Kit and method for detecting aminoglycoside drug-induced deafness-sensitive gene
A technology of drug-induced deafness and aminoglycosides, which is applied in the fields of biochemical equipment and methods, and microbial measurement/inspection, and can solve the problems of long operating time of microarray chip products, inability to perform quality control, and the need for special instruments, etc. Achieve the effect of high cost, fast detection and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The preparation and use of embodiment 1 kit of the present invention
[0063] The kit of the invention comprises PCR reaction solution and quality control products. The PCR reaction solution includes Taq enzyme, DNA double-stranded chimeric dye, amplification primer, amplification buffer, dN(U)TP, etc., and dN(U)TP is the dNTP mixture. The PCR reaction program is run on a fluorescent PCR instrument and the detection results are read.
[0064] 1. Design of primers for the detection of susceptibility genes for aminoglycoside-induced deafness
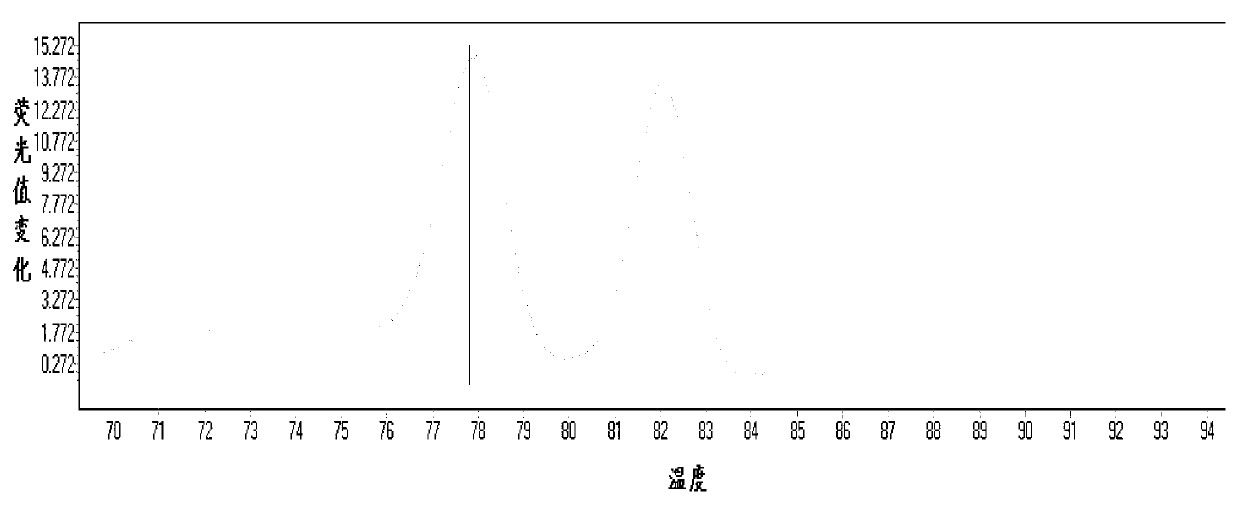
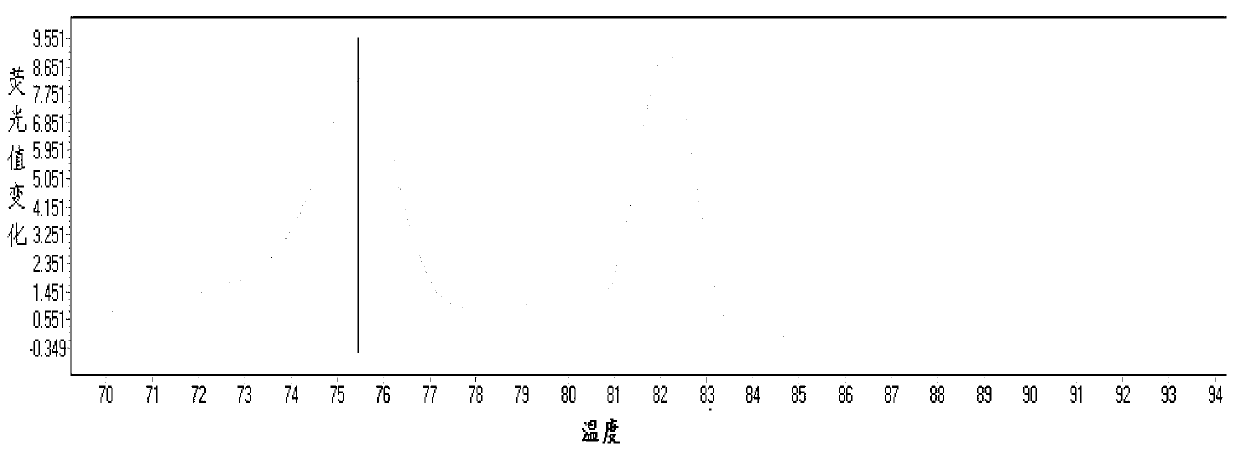
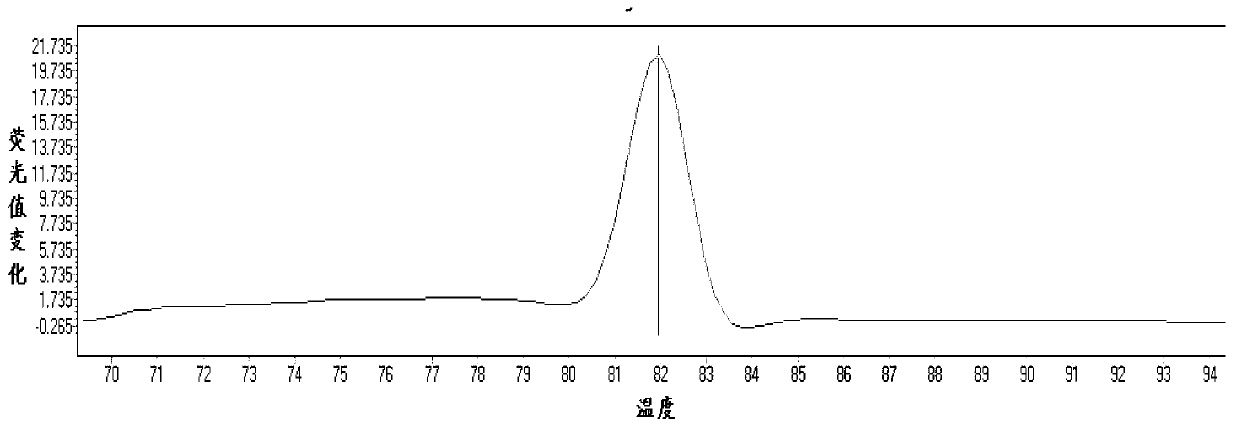
[0065] According to the principle of site-specific primer design, the mutation site was set as the 3' end of the primer, and point mutations were introduced at the 2nd, 3rd, and 4th positions of the 3' end to improve specificity. Finally, 5 usable site-specific primers were selected from 20 primers, including two for 1555 site and three for 1494 site. The position of the mutation site is crucial to the specificity of the primer. ...
Embodiment 2
[0092] The use effect of embodiment 2 kit of the present invention
[0093] 1, the comparison of kit of the present invention and existing similar technology
[0094] Compared with the prior art, the present invention establishes a screening method for sites of aminoglycoside antibiotic drug-induced deafness, which is suitable for rapid detection and mass screening. Only one tube reaction can detect two mitochondrial mutation sites popular in the Chinese population, avoiding the problems of cumbersome operations, cross-contamination, and low accuracy.
[0095] 2. Detection of C1494T and A1555G mutations in clinical samples using the kit of the present invention
[0096] This kit was used to detect 200 samples from the general population, and to detect the C1494T and A1555G mutations in clinical samples. All samples were sequenced and verified by the gold standard sequencing method. The detection results and sequencing results of the kit of the present invention and their comp...
Embodiment 3
[0120] Embodiment 3 A specific embodiment of the kit of the present invention
[0121] 1. The main components of the kit of the present invention are as shown in Table 6
[0122]
[0123] Table 6
[0124] 2. Applicable instruments
[0125] Including ABI Prism 7300 / 7500 / 7700 / 5700 / 7000 / 7900, Roche LightCycler 480 and other fluorescent PCR amplification detectors.
[0126] 3. Storage conditions and validity period
[0127] Storage conditions: The kit should be stored at -20°C protected from light.
[0128] Validity period: 6 months.
[0129] 4. Sample requirements
[0130] (1) Sample type
[0131] Dried blood film, anticoagulated peripheral blood, oral swab.
[0132] (1)Sample storage
[0133] Store at -20°C for no more than one year.
[0134] (2) Specimen transportation
[0135] Dried blood spots and oral swabs can be transported at room temperature, and blood samples are transported sealed with ice.
[0136] (3) Sample size
[0137] The upper limit of detection i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com