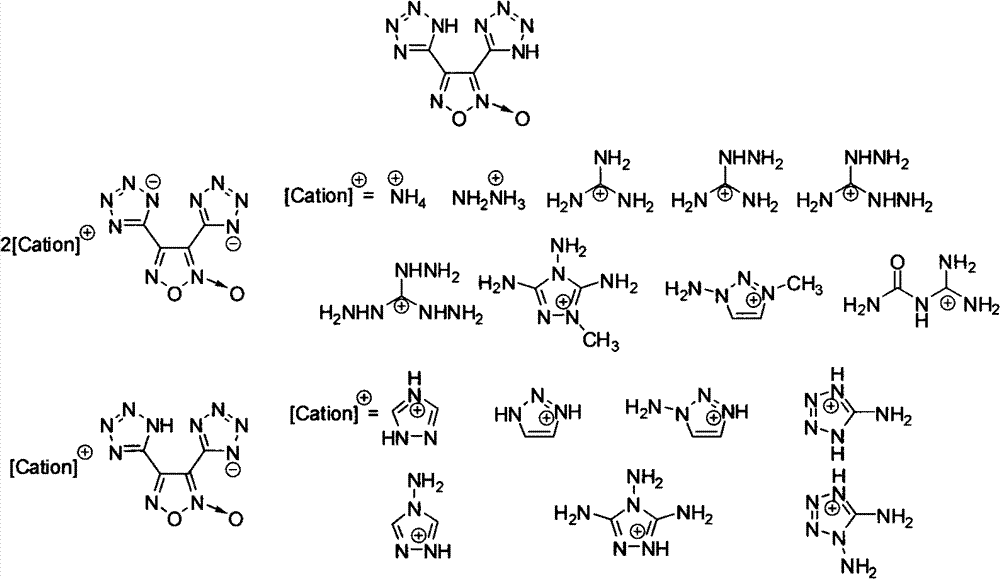
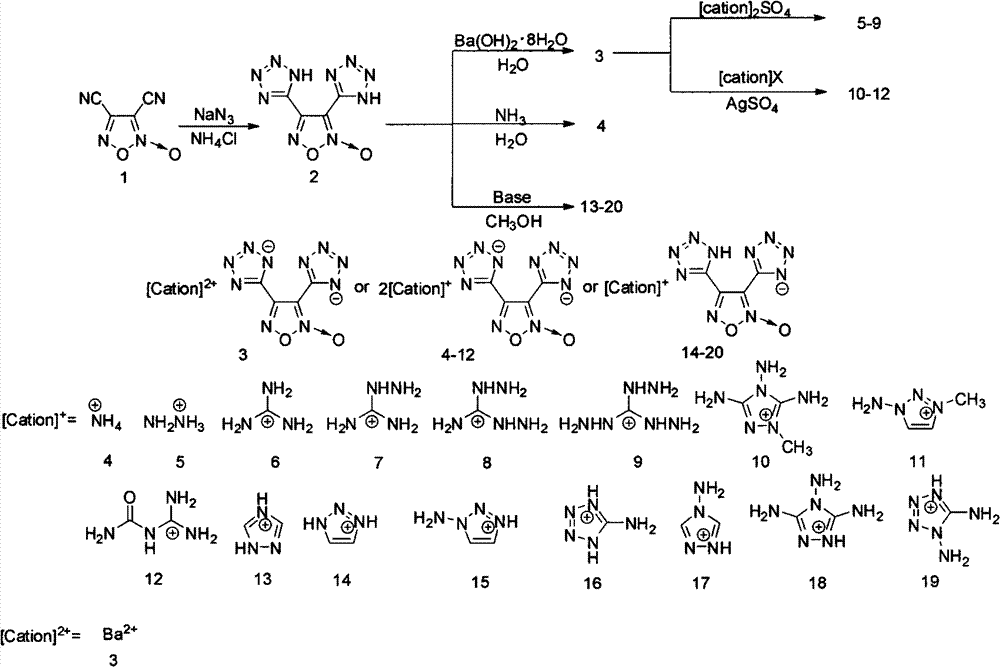
3,4-bis(1-hydro-5-tetrazolyl)furoxan ionic salt containing energy and preparation method thereof
A technology for oxidizing furoxan and tetrazolium, which is applied in 3 fields and achieves the effects of easy industrialization, low impact sensitivity and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
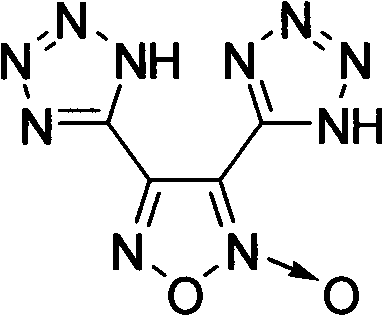
[0021] Embodiment 13, the preparation of 4-bis (1-hydrogen-5-tetrazolyl) furazan oxide (2)
[0022] Its structural formula is as follows:
[0023]
[0024] Dissolve 1.000 g (7.3 mmol) of 3,4-dicyanofuroxan in 4 mL of DMF in a 25 mL one-necked flask, and then add 1.147 g (17.6 mmol) of NaN 3 and 0.786g (14.7mmol). The resulting reaction mixture was stirred at 80°C for 4 h and then cooled to room temperature. After adding 4 mL of deionized water, the pH was adjusted to 1-2 with 2% hydrochloric acid, extracted with ethyl acetate (10 mL×4), and the organic phases were combined and saturated. washed several times with saline, and the organic phase was washed with anhydrous MgSO 4 After drying, it was concentrated to give a pale yellow solid. Yield: 60%.
[0025] Decomposition temperature: 229°C (DSC). Density is 1.62g cm -3 . 13 C NMR: δ=149.0, 146.3, 144.5, 106.8ppm; IR (neat): 3135, 2908, 2446, 1620, 1579, 1457, 1418, 1386, 1282, 1232, 1203, 1181, 1128, 1093, 1069, 1025...
Embodiment 23
[0026] Example 23, 4-bis(5-tetrazolyl) furazan barium salt 8H 2 Preparation of O(3)
[0027] Its structural formula is as follows:
[0028]
[0029] Add 2.000g (9.0mmol) 2 to 2.839g Ba(OH) 2 ·8H 2 In a suspension of O (9.0 mmol) in water (20 mL), the reaction mixture was stirred at 80° C. for 2 h, then the insoluble matter was filtered, and the filtrate was concentrated. The resulting solid was recrystallized in water as yellow crystals, yield: 80%.
[0030] Decomposition temperature: 297°C (DSC). Density is 2.07g cm -3 . 13 C NMR: δ=152.4, 150.5, 147.0, 111.0ppm; IR (neat): 3566, 3329, 3172, 1602, 1574, 1532, 1440, 1387, 1367, 1394, 1238, 1203, 1188, 1148, 1110, 1095 , 1064, 1044, 1003, 971, 827, 763, 534, 481cm -1 .
Embodiment 33
[0031] Embodiment 33, the preparation of 4-bis(5-tetrazolyl) oxide furazan diammonium salt (4)
[0032] Add excess 25% ammonia water to 222mg (1.0mmol) methanol (3mL) solution, stir at room temperature for 2h, then evaporate the solvent under reduced pressure, the yield is 95%, and the obtained solid is washed with EtOH / H 2 O Recrystallized colorless needle crystals.
[0033] Its structural formula is as follows:
[0034]
[0035] Decomposition temperature: 262°C (DSC). Density is 1.71g cm -3 . 1 H NMR (400MHz, d 6 -DMSO): δ = 7.29 (s, 8H) ppm; 13 C NMR (100MHz, d 6 -DMSO): δ=152.1, 150.5, 147.0, 110.8ppm; IR (neat): 3137.8, 3012.5, 2804.1, 1680.6, 1611.8, 1579.4, 1527.3, 1501.8, 1405.0, 993.8, 966.1, 824.3cm -1;elemental analysis(%) calcd for C 4 h 8 N 12 o 2 (256.19): C 18.75, H 3.15, N 65.61; found: C 18.81, H 3.02, N 65.05.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Decomposition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com