Febuxostat tablets and preparation method and detection method thereof
A technology of Buxostat tablets and Febuxostat, which is applied in the field of western medicine and can solve problems affecting the curative effect of Febuxostat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
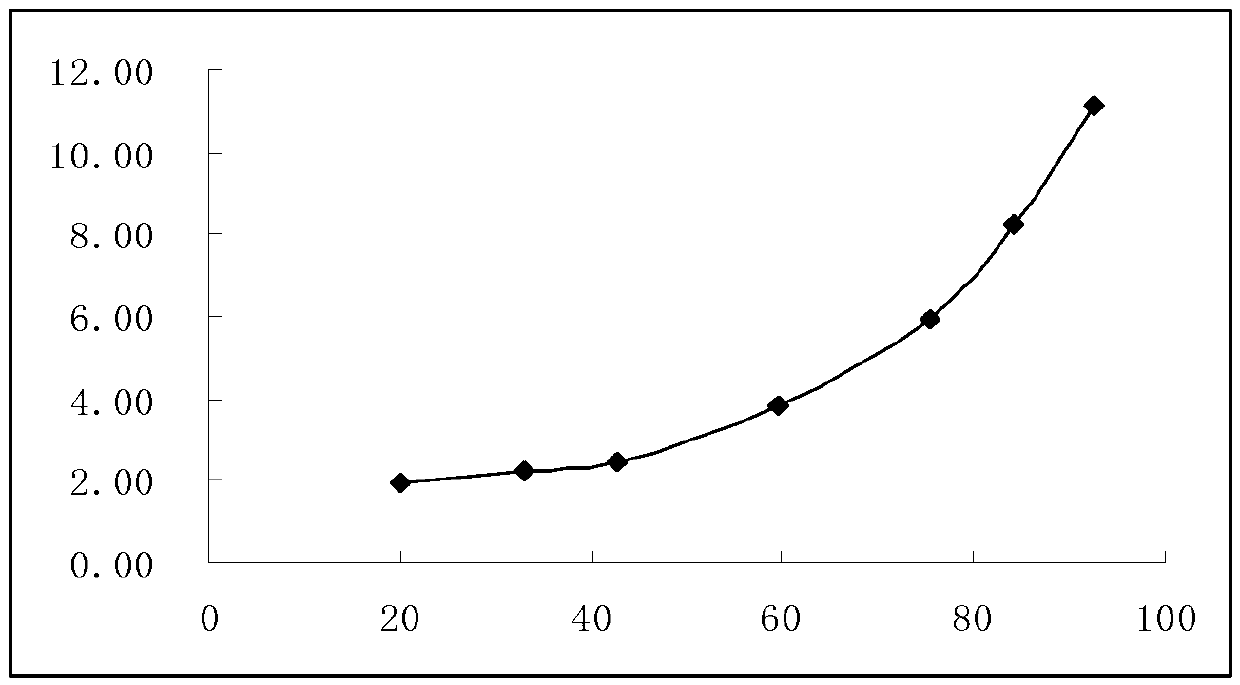
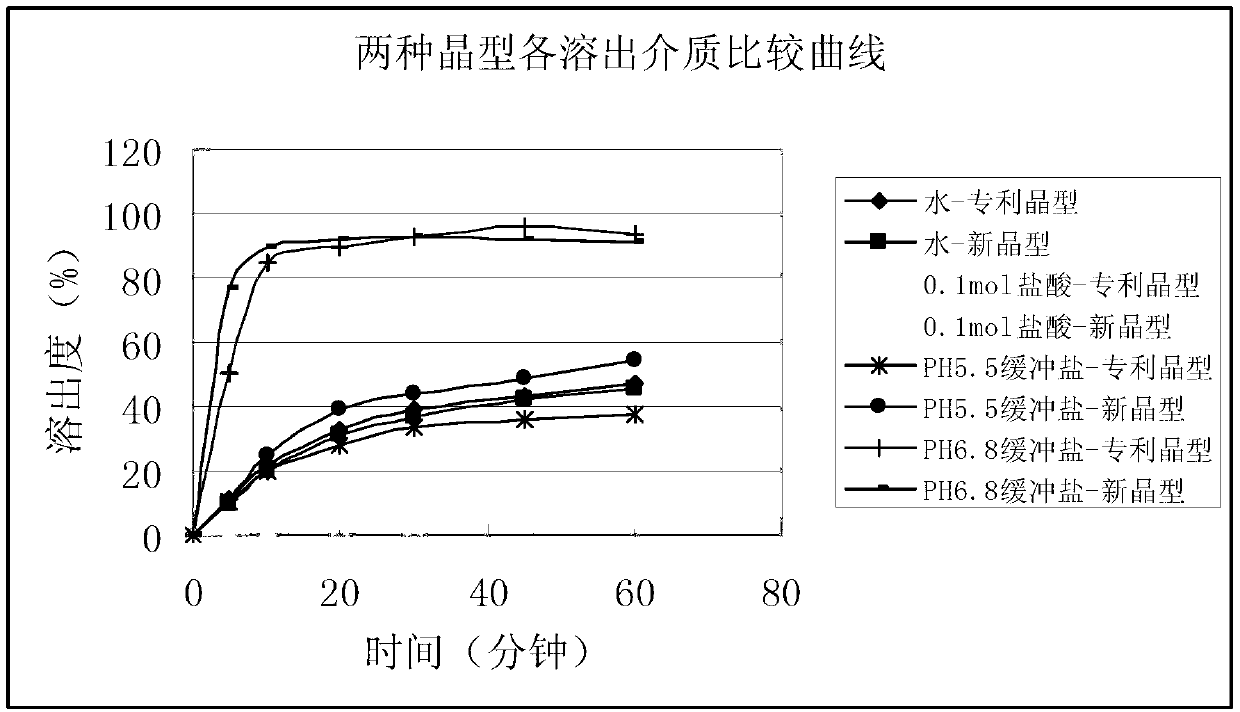
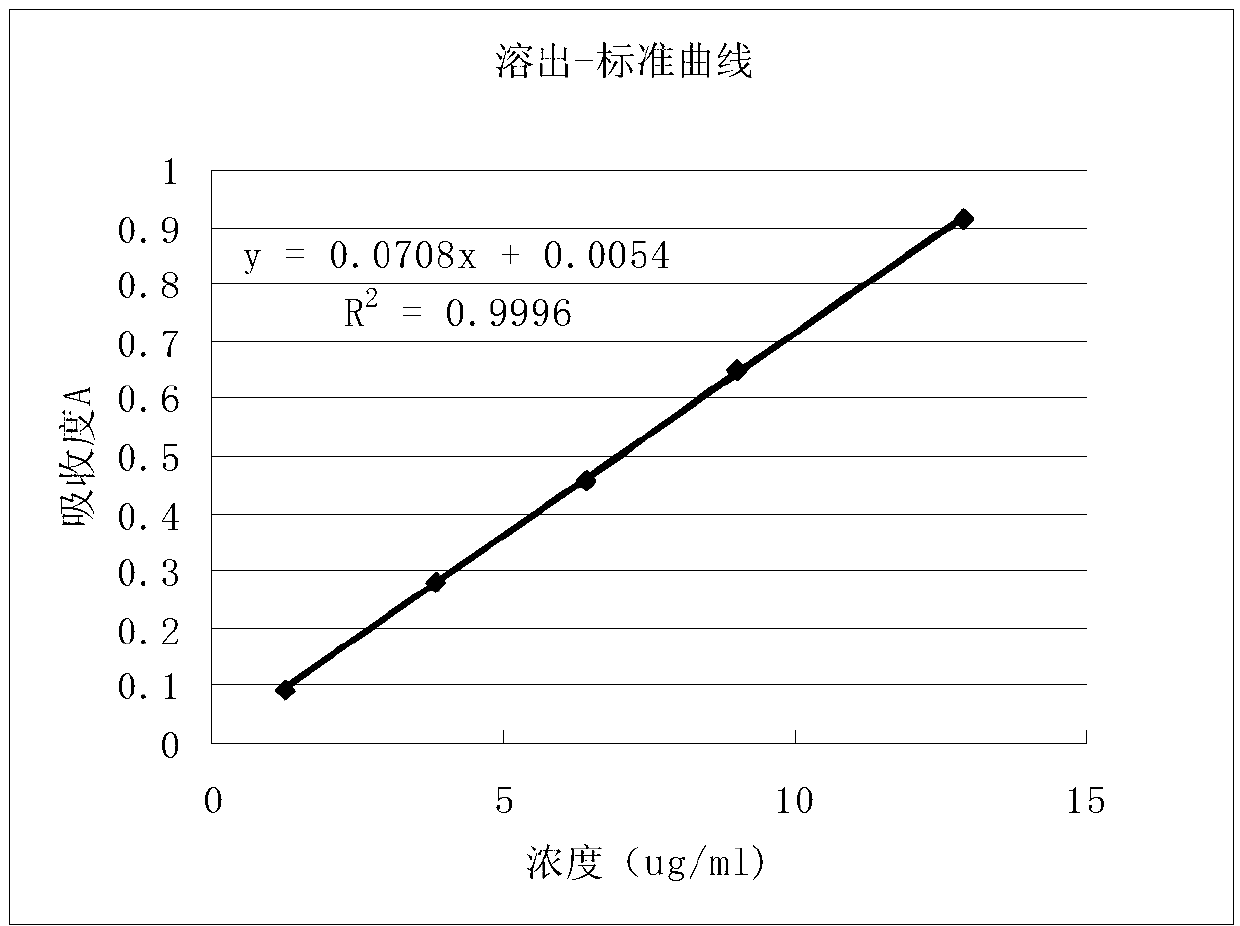
Image
Examples
Embodiment 1
[0236] Embodiment 1: The febuxostat tablet is made of 80g febuxostat, 110g microcrystalline cellulose, 40g lactose, 18g croscarmellose sodium and 2g magnesium stearate. Take febuxostat and lactose, pass through 80-mesh sieve respectively, and set aside; mix 80g febuxostat with 110g microcrystalline cellulose, 40g lactose, and 8g croscarmellose sodium, and add appropriate amount of purified water to prepare Soft material, granulated with 18-mesh sieve, dried at 70°C, granulated with 18-mesh sieve, added the remaining amount of croscarmellose sodium and 2g magnesium stearate, mixed evenly; tested for intermediates, pressed into tablets after passing the test, and obtained Vegetable tablets: Take 370g ethanol and 100g purified water and place them in a blender, add 30g premixed coating powder under constant stirring, continue to stir for 1 hour, filter the coating solution with a 100-mesh sieve, and set aside; Put the tablet in the coating pot, open the coating pot and start the ...
Embodiment 2
[0237] Embodiment 2: the febuxostat tablet is made of 120g febuxostat, 165g microcrystalline cellulose, 60g lactose, 27g croscarmellose sodium and 3g magnesium stearate. Take febuxostat and lactose, pass through 80-mesh sieve respectively, and set aside; mix 120g febuxostat with 165g microcrystalline cellulose, 60g lactose, and 12g croscarmellose sodium, and add appropriate amount of purified water to prepare Soft material, granulated with 18-mesh sieve, dried at 70°C, granulated with 18-mesh sieve, added the remaining amount of croscarmellose sodium and 3g magnesium stearate, mixed evenly; tested for intermediates, pressed into tablets after passing the test, and obtained Vegetable tablets: Take 550g ethanol and 150g purified water and place them in a blender, add 45g premixed coating powder under constant stirring, continue to stir for 1 hour, filter the coating solution with a 100-mesh sieve, and set aside; Put the tablet in the coating pot, open the coating pot and start t...
Embodiment 3
[0238] Embodiment 3: the febuxostat tablet is made of 40g febuxostat, 95g microcrystalline cellulose, 20g lactose, 10g croscarmellose sodium and 1g magnesium stearate. Take febuxostat and lactose, pass through 80-mesh sieve respectively, and set aside; mix 40g febuxostat with 95g microcrystalline cellulose, 20g lactose, 4g croscarmellose sodium, add appropriate amount of purified water to prepare Soft material, granulated with 18-mesh sieve, dried at 70°C, granulated with 18-mesh sieve, added the remaining amount of croscarmellose sodium and 1g magnesium stearate, mixed evenly; tested for intermediates, pressed into tablets after passing the test, and obtained Vegetable tablet: take 185g ethanol, 50g purified water and place in a blender, add 15g premixed coating powder under the condition of constant stirring, continue to stir for 1 hour, and filter the coating solution with a 100 mesh screen, set aside; Put the tablet in the coating pot, open the coating pot and start the ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com