Dimethyl carbonate synthesis method by using methanol and carbon dioxide
A technology of dicyclohexylcarbodiimide and methanol, which is applied to the preparation of carbon dioxide or inorganic carbonate, chemical instruments and methods, nickel organic compounds, etc. It can solve the problems of difficult activation, reaction thermodynamic limitations, and low reaction conversion rate. , to achieve the effects of less dosage, chemical transformation and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Preparation and characterization of catalyst
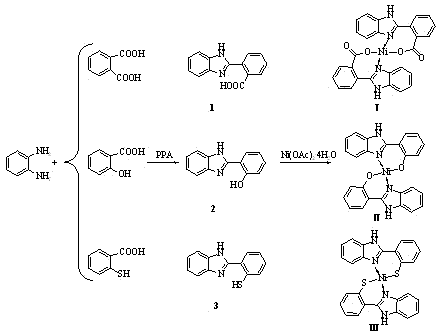
[0022] Catalyst of the present invention is benzimidazole derivative ligand and Ni(OAc) 2 . 4H 2 The complex generated by the O reaction, wherein the benzimidazole derivative ligand is synthesized according to the method reported in the literature, that is, it is prepared by condensation of o-phenylenediamine and the corresponding benzoic acid derivative, and the preparation route of the catalyst is as follows figure 1 shown.
[0023] Preparation of Catalyst I
[0024] Ligand 1 Synthesis
[0025] Add 10.80 g of o-phenylenediamine and 16.6 g of phthalic acid into a 250 ml round bottom flask, then add an appropriate amount of PPA (polyphosphoric acid), slowly raise the temperature, stir and heat, and start timing when the temperature rises to 150 °C. After 4 h, the reaction solution was emerald green. After the reaction was over, it was poured into a 500 ml beaker filled with cold water, stirred thorou...
Embodiment 2
[0038] Embodiment 2: Methanol and carbon dioxide synthesis dimethyl carbonate catalyst screening
[0039] In 250 ml autoclave, add corresponding catalyst (catalyst 1~ Ⅲ ) 1 mmol, 2.06 g DCC and 32 g methanol, the CO 2 After replacing the reactor three times, control the pressure to 1.0 MPa, stir and heat, and start timing when the temperature rises to 70 °C. After reacting for 6 hours, cool the reactor to room temperature, distill the reaction liquid, and use GCMS to collect the liquid Detect the content of dimethyl carbonate (using n-butanol as internal standard), and calculate the reaction yield (in DCC). The results are shown in Table 1, the catalyst Ⅰ The catalytic effect is the best.
[0040]
Embodiment 3
[0041] Embodiment 3: take DCC as coupling agent, catalyst Ⅰ is the catalyst, methanol and carbon dioxide synthesis of dimethyl carbonate reaction temperature screening
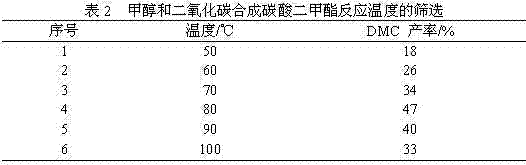
[0042] Similar to Example 2, the difference is: methanol and carbon dioxide synthesize dimethyl carbonate, with DCC as coupling agent, catalyst Ⅰ As a catalyst, the reaction temperature is from 50-100°C. The results are shown in Table 2, the optimum reaction temperature is 80°C.
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com