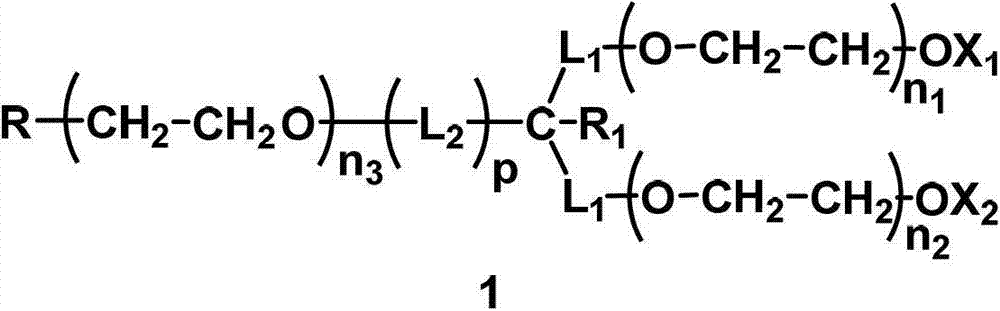
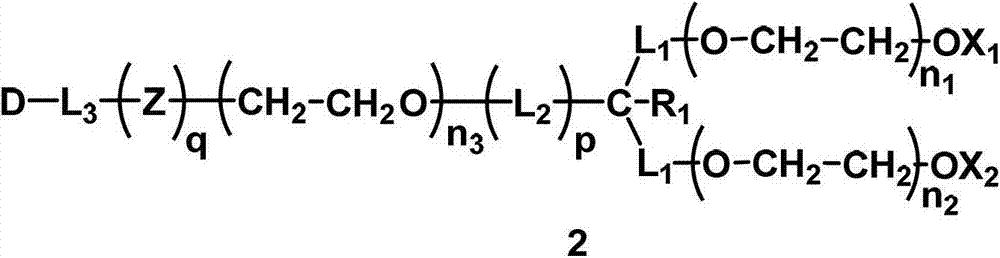
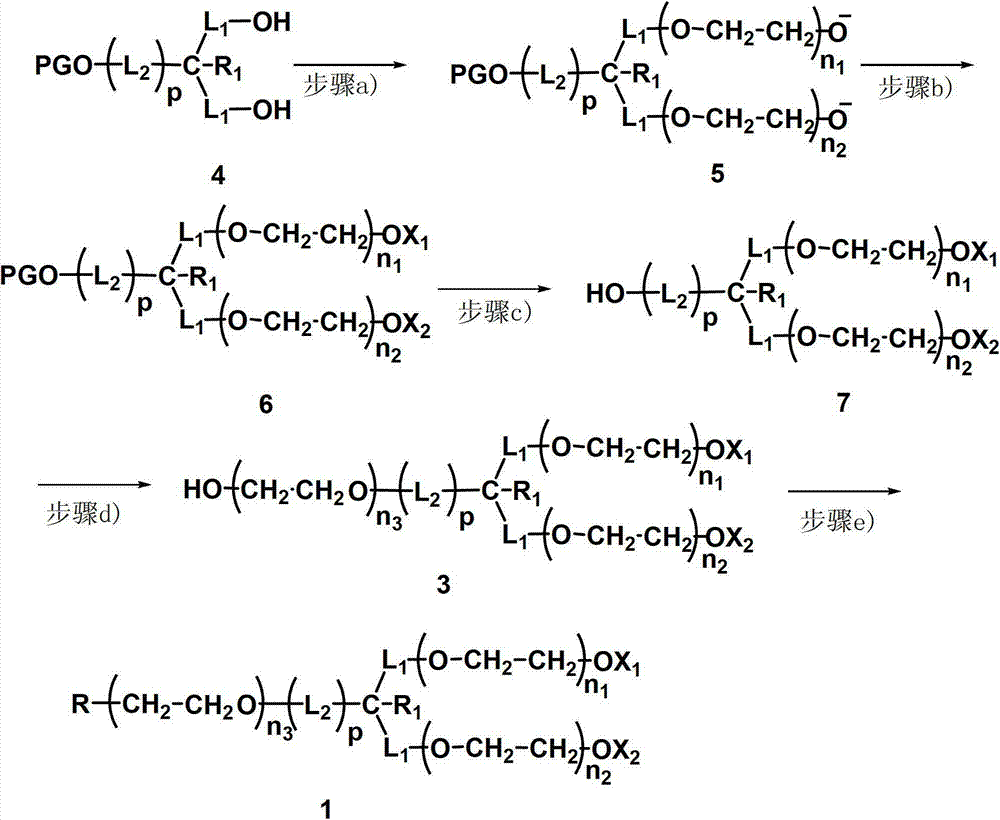Monofunctional branched polyethyleneglycol
A polyethylene glycol and functionalization technology, which is applied in organic chemistry, peptide preparation methods, chemical instruments and methods, etc., can solve the problems of inability to perform specific reactions, reduce the difficulty of purification, reduce steric hindrance, and improve modification rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] A kind of preparation method of the branched polyethylene glycol of described single functionalization, comprises the steps:
[0074] a) A co-initiation system is composed of a small molecule initiator (4) containing a symmetrical hydroxyl group and potassium benzhydryl to initiate the polymerization of ethylene oxide, generate two branched chains, and deprotonate the ends of the branched chains to obtain an intermediate (5);
[0075] b) Capping the two branched chains of the intermediate (5) obtained in step a) to obtain the intermediate (6);
[0076] c) deprotecting the terminal hydroxyl group of the symmetry axis of the intermediate (6) obtained in step b) to obtain the intermediate (7);
[0077] d) Polymerizing ethylene oxide on the hydroxyl group of the symmetrical axis end of the intermediate (7) obtained in step c), forming a main chain of the symmetrical axis, and obtaining the intermediate (3) after protonation;
[0078] e) The intermediate (3) obtained in st...
Embodiment 1
[0270] Embodiment 1: R is the preparation of monofunctional branched polyethylene glycol when class H
[0271] Preparation of Compound H1-1
[0272] In this example, the H-like compound selected L 1 =CH 2 , L 2 =CH 2 , R 1 =H,X 1 =X 2 =CH 3 , p=1, q=0, the protecting group PG=TBS of the hydroxyl group at the end of the symmetry axis of the small molecule initiator. The total molecular weight is designed to be about 20000, and the molecular weight of the two branched chains is about 2*8500=17000, namely n 1 ≈n 2 ≈193; the molecular weight of the main chain of the symmetry axis is about 3000, that is, n 3 ≈68.
[0273]
[0274] a. Add tetrahydrofuran (250mL), small molecule initiator (2.532mmol) and diphenylmethyl potassium (4.0mmol) sequentially into an anhydrous and oxygen-free airtight reaction kettle;
[0275] b. Add the calculated amount of ethylene oxide (50mL), gradually raise the temperature to 60°C, and react for 48 hours;
[0276] c. Add excess diphenyl...
Embodiment 2
[0319] The preparation of embodiment 2 active ester derivatives
[0320] Synthesis of Active Ester A1-1
[0321] Synthesis of active ester (A1-1), where L 1 =CH2 , L 2 =CH 2 , R 1 =H,X 1 =X 2 =CH 3 , Z is OCH 2 CH 2 O, p=1, q=1, the molecular weight is about 20000, where n 1 , n 2 , n 3 The value of is the same as that of compound H1-1. In this example, the hydroxyl group at the end of the main chain of the symmetry axis of compound H1-1 is directly reacted with carbonate to prepare the corresponding active ester.
[0322]
[0323] In a dry and clean 1L round bottom flask, add 40g of branched polyethylene glycol (H1-1, dewatered by azeotropic toluene) prepared in Example 1, 500mL of acetonitrile, 40mL of triethylamine and 10g of N,N' - Disuccinimidyl carbonate, reacted at room temperature for 24 hours, concentrated, and recrystallized from isopropanol to obtain the active ester (A1-1) as a white solid.
[0324] The hydrogen spectrum data of active ester A1-1 i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com