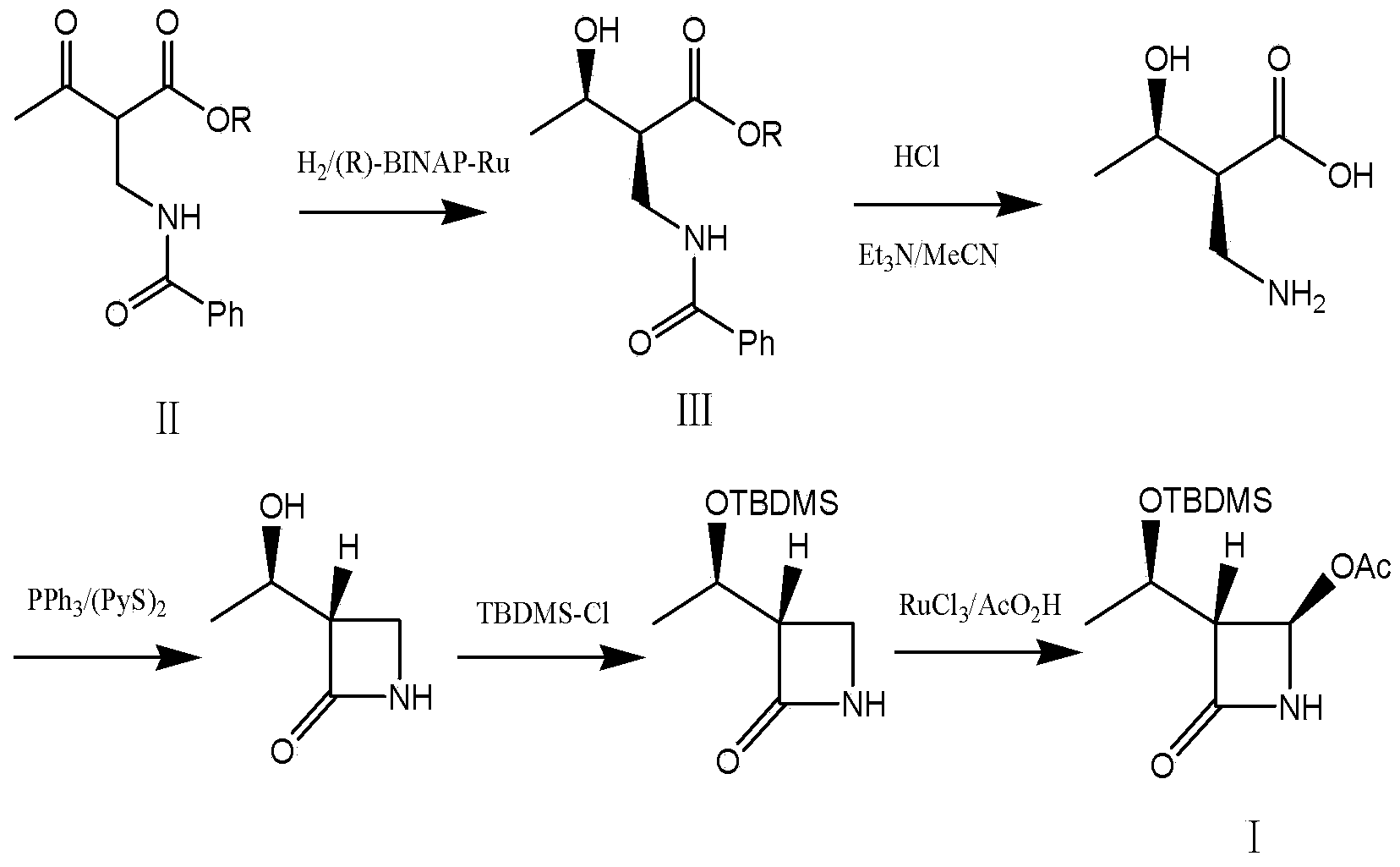
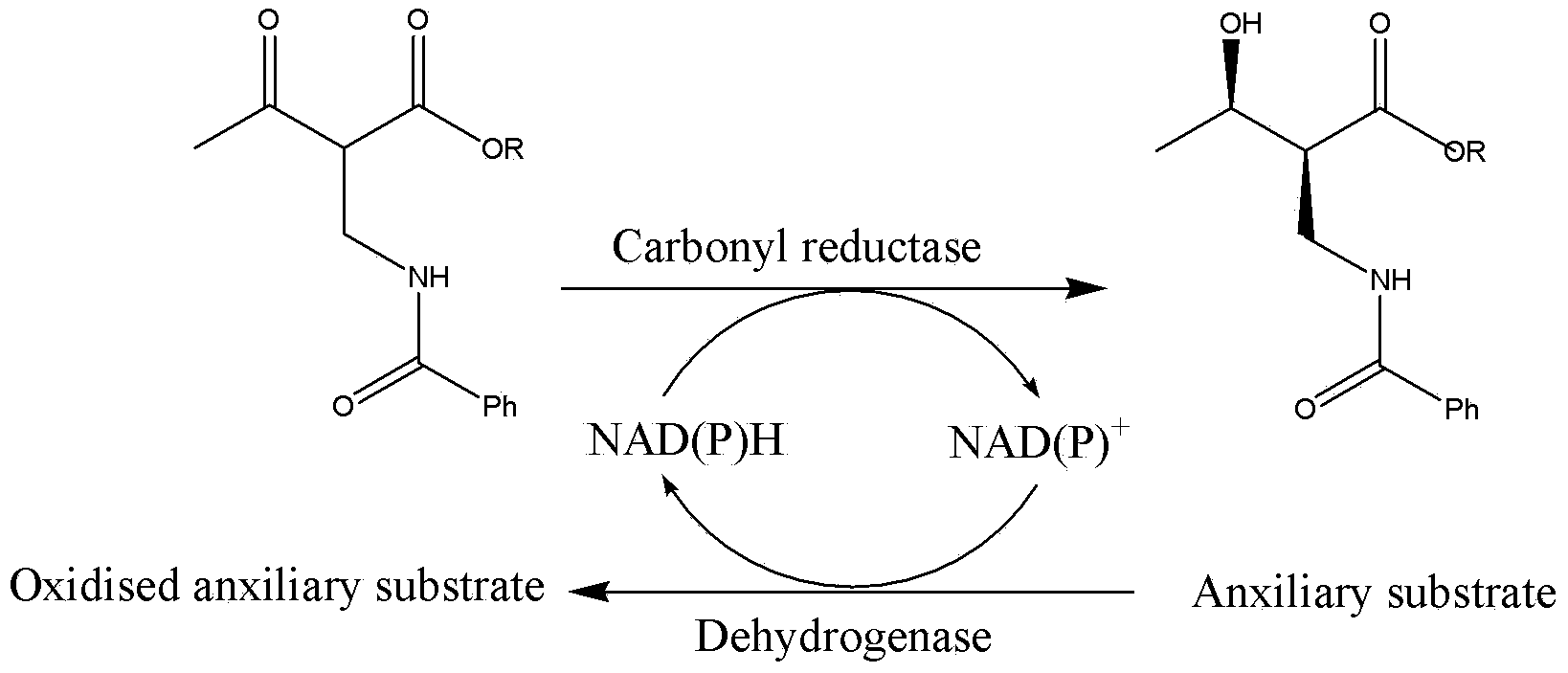
Microorganism catalysis prepared (2S,3R)-2-benzoyl aminomethyl-3-hydroxybutyric acid ester and bacterial strain
A technology of benzoylaminomethyl and hydroxybutyrate, which is applied in the field of microbial catalytic preparation of (2S, 3R)-2-benzoylaminomethyl-3-hydroxybutyrate and strains, and can solve the problem of 2- Benzoylaminomethyl-3-hydroxybutyrate has problems such as few reports, low recovery rate, and complicated operation, which achieves good industrial application prospects, mild reaction conditions, and simple operation procedures.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Screening, identification of microorganisms
[0042] (1) Screening of microorganisms
[0043] Strain enrichment medium: glucose 20g / L, yeast extract 10g / L, peptone 20g / L, 2-benzamidomethyl-3-oxobutyric acid methyl ester 40g / L, dimethyl sulfoxide 40mL / L, the solvent is water, pH 7.0.
[0044] Plate medium and slant medium: glucose 20g / L, yeast extract 10g / L, peptone 20g / L, agar 20g / L, solvent is water, pH 7.0.
[0045] Seed solution medium: glucose 20g / L, yeast extract 10g / L, peptone 20g / L, solvent is water, pH 7.0.
[0046] Fermentation enzyme production medium: glucose 30g / L, yeast extract 30g / L, NaCl 4g / L, solvent is water, pH 7.0.
[0047] More than 200 soil samples were collected from vegetable orchard plant clusters in Zhejiang, Guangdong, Shandong, Liaoning and Jiangsu for strain screening. The specific process is as follows:
[0048] Take a little soil sample and add it to physiological saline to make a soil suspension, which is enriched twice...
Embodiment 2
[0062] Example 2: Preparation of wet thallus of Burkholderia gladiolus ZJB-12126
[0063] (1) Slant culture: Burkholderia gladioli ZJB-12126 was inoculated on the slant medium and cultured at 30°C for 24 hours to obtain slant cells. The formula of the slant medium is: glucose 20g / L, yeast extract 10g / L, peptone 20g / L, agar 20g / L, the solvent is water, and the pH is 7.0.
[0064] (2) Seed cultivation: pick a ring of bacteria from the slant with an inoculation loop and inoculate it in the seed medium, and cultivate it at 30°C and 150r / min for 16 hours to obtain the seed liquid. The formula of the seed medium is: glucose 20g / L, yeast extract 10g / L, peptone 20g / L, the solvent is water, and the initial pH is 7.0.
[0065] (3) Fermentation culture: Inoculate the cultured seed liquid into the fermentation medium at a volume ratio of 2% to obtain bacteria. The formula of the fermentation medium is: glucose 20g / L, yeast extract 10g / L, NaCl 2g / L, the solvent is water, the initial pH i...
Embodiment 3
[0066] Example 3: Preparation of wet thallus of Burkholderia gladiolus ZJB-12126
[0067] (1) Incline cultivation: same as in Example 2.
[0068] (2) Seed culture: same as Example 2.
[0069] (3) Fermentation culture: Inoculate the cultured seed liquid into the fermentation medium at a volume ratio of 5% to obtain bacteria. The formula of the fermentation medium is: glucose 30g / L, yeast extract 30g / L, NaCl 5g / L, the solvent is water, the initial pH is 7.0, cultivated at 30°C and the shaker rotation speed 150r / min for 24 hours to obtain Fermentation broth containing bacterial cells. The fermentation broth was centrifuged, and the supernatant was discarded. After the precipitate was washed twice with physiological saline, the precipitate was collected by centrifugation to obtain wet thallus. The yield of the wet thallus was 83 g / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com