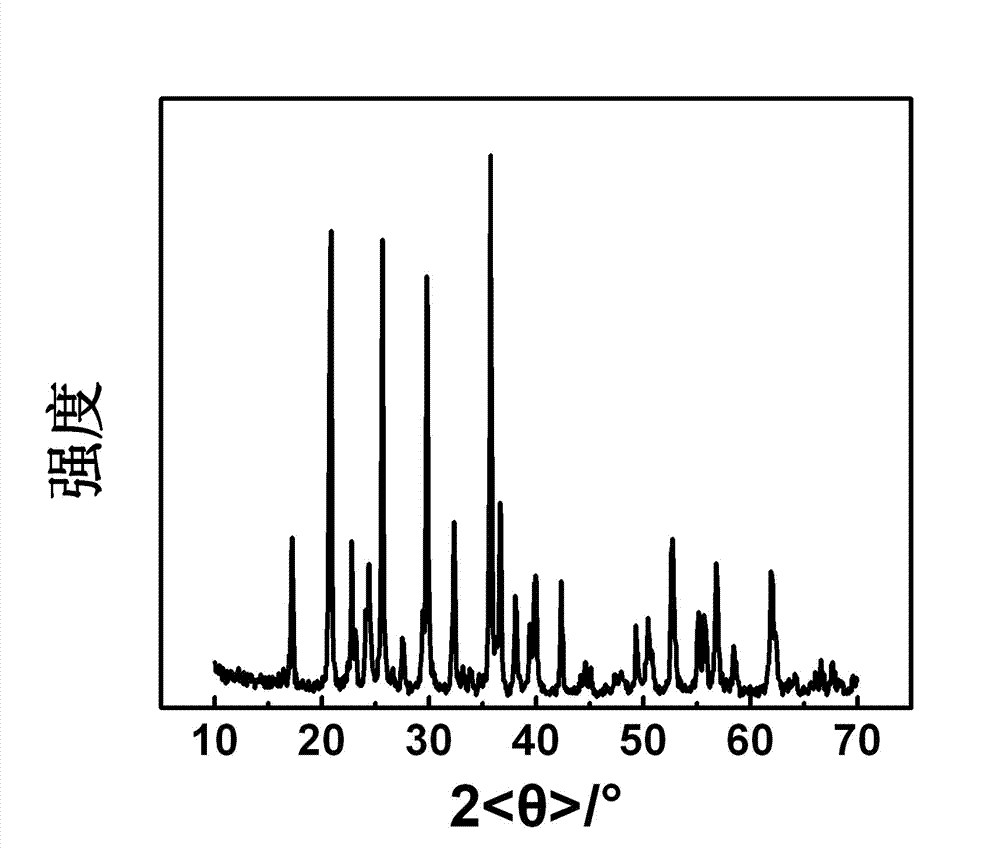
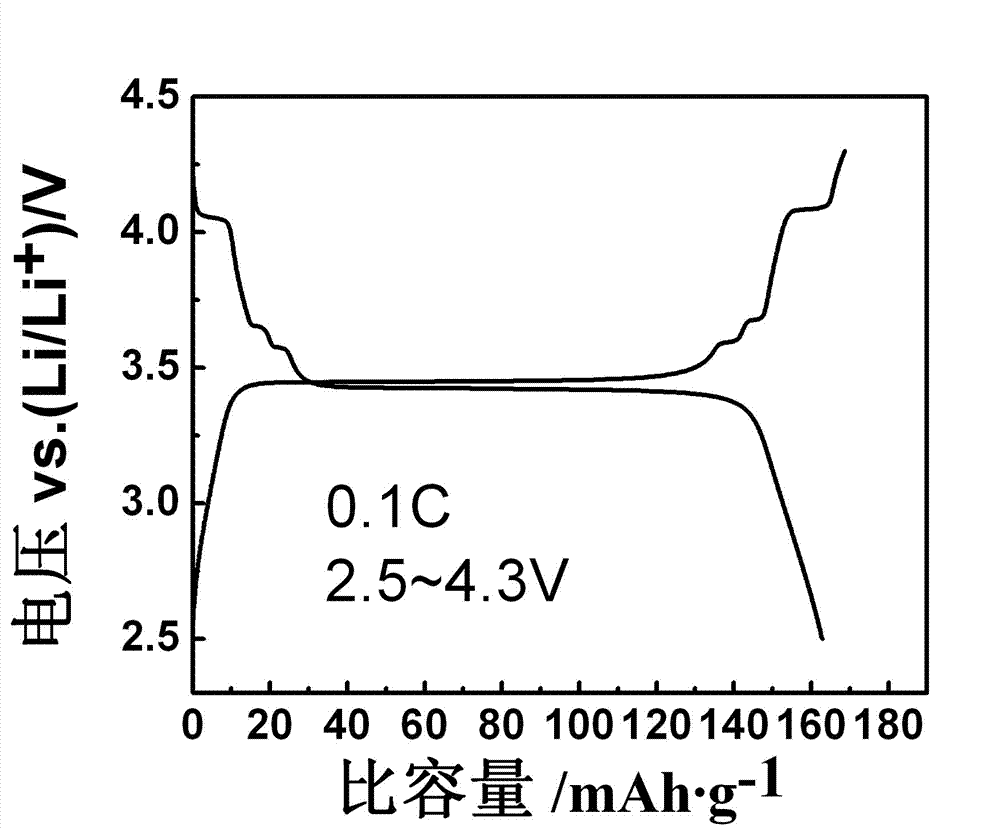
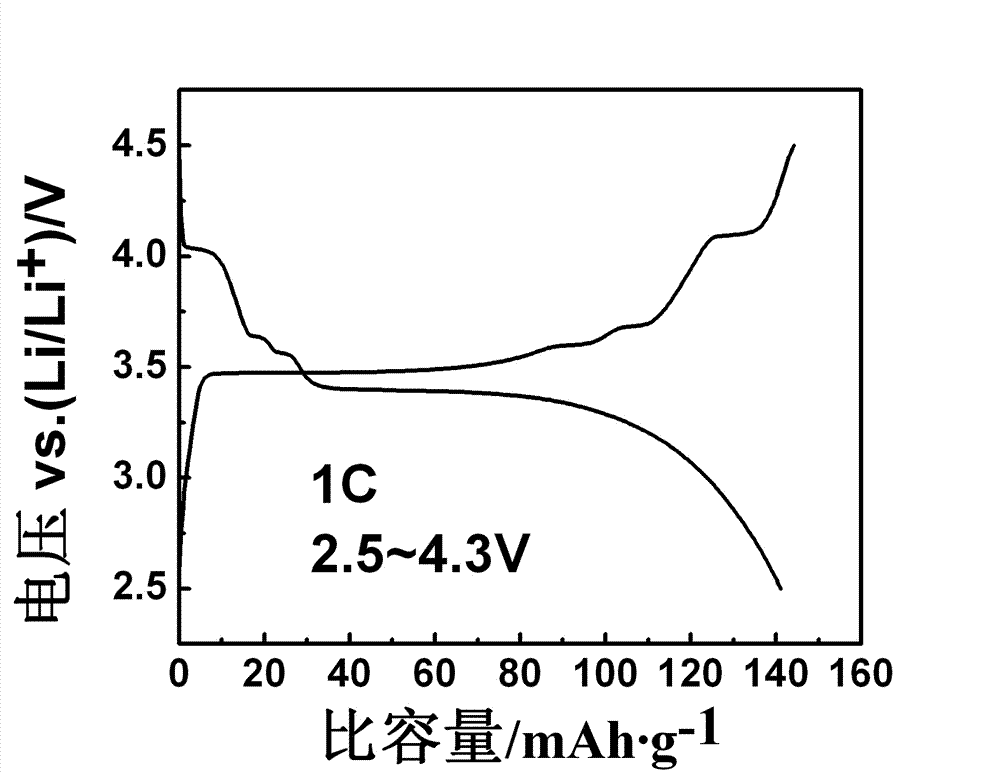
Vanadium lithium iron phosphate anode material and preparation method thereof
A technology of lithium vanadium iron phosphate and positive electrode materials, applied in chemical instruments and methods, phosphorus compounds, battery electrodes, etc., can solve problems such as difficulty in deintercalation and poor cycle performance of materials, and achieve performance improvement, structural stability and cycle performance. good performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Take Li x Fe 1-3y V 2y PO 4 In / C x=1.06, y=0.1. Weigh Li according to the molar ratio Li:Fe:V:P=1.06:0.7:0.2:1 2 CO 3 , FeC 2 o 4 2H 2 O, NH 4 VO 3 and NH 4 h 2 PO 4, while weighing 10% of the theoretical product mass of citric acid as a carbon source, weighing 0.1% of the starting material mass of polyethylene glycol as a dispersant. Add ethanol as the liquid phase medium according to 1 times the mass of the starting material, and at the same time add zirconium balls with a diameter of 3 μm according to the ball-to-material ratio of 1:1, and ball mill at 175 r / min for 3 hours. After the ball-milled sample was vacuum-dried at 120°C for 10 hours, it was pre-fired at 380°C for 4 hours in a tube furnace fed with nitrogen, and after cooling to room temperature, the sample was ground for a second time and then heated at 650°C , and nitrogen atmosphere for 18h sintering, and then cooled to room temperature, the lithium vanadium iron phosphate positive electrode...
Embodiment 2
[0030] Take Li x Fe 1-3y V 2y PO 4 In / C x=1.02, y=0.1. Weigh LiOH·H according to the molar ratio Li:Fe:V:P=1.02:0.7:0.2:1 2 O, FeC 2 o 4 2H 2 O, NH 4 VO 3 and (NH 4 ) 2 HPO 4 , while weighing 8% of the theoretical product mass of sucrose as a carbon source, weighing 3% of the starting material mass of polyacrylamide as a dispersant. Add acetone as the liquid phase medium according to the mass ratio of the starting material 1.2:1, and add 2 μm steel balls according to the ball-to-material ratio of 4:1, and ball mill at 220r / min for 2h. After the ball milled sample was dried at 50°C for 12 hours, it was pre-fired at a low temperature of 300°C for 6 hours in a tube furnace fed with argon gas, then cooled, and after regrinding, it was sintered at 700°C for 16 hours, and cooled to room temperature to obtain ferrovanadium phosphate Lithium cathode material.
Embodiment 3
[0032] Take Li x Fe 1-3y V 2y PO 4 In / C x=1.1, y=0.2. Weigh LiOH·H according to the molar ratio Li:Fe:V:P=1.1:0.4:0.4:1 2 O, Fe 2 o 3 , V 2 o 5 and (NH 4 ) 2 HPO 4 At the same time, glucose with 15% of the theoretical product mass was weighed as a carbon source. Weigh 1.5% polyethylene glycol by weight of the starting material as a dispersant. Add deionized water as the liquid phase medium according to the mass ratio of starting materials 5:1, and add 3 μm steel balls according to the ball-to-material ratio of 8:1, and ball mill at 300 r / min for 0.5 h. The ball-milled samples were dried in a vacuum oven at 100°C for 24 hours, and then the samples were finely ground. Pre-fire the ground sample in a tube furnace with reducing gas (2% hydrogen + 98% nitrogen) at a low temperature of 300°C for 6 hours, directly raise the temperature to 750°C for sintering for 12 hours, cool to room temperature and grind to obtain ferrovanadium phosphate Lithium cathode material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com