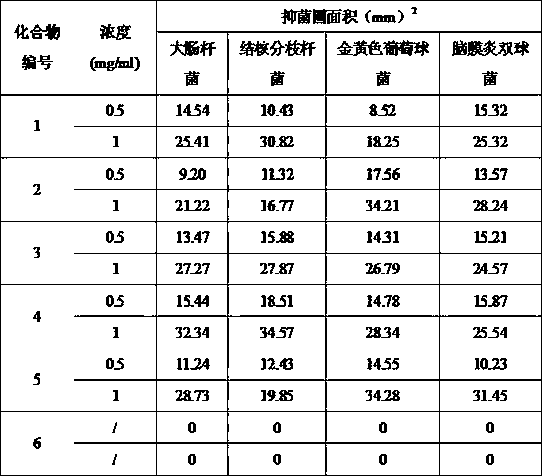
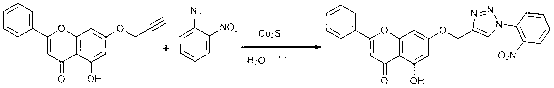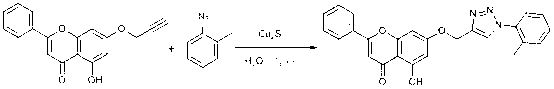Chrysin-1,2,3-triazole compound having antibacterial activity, and its preparation method
A technology of antibacterial activity and chrysin, applied in botany equipment and methods, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low activity, achieve high product yield, simple process, excellent antibacterial performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) 5-hydroxy-2-phenyl-7-(propyl-2-ynyl-1-yloxy)-4 H - Preparation of chroman-4-one
[0020]
[0021] Chrysin (30 mmol, 7.63 g) and 3-bromopropyne (30 mmol, 3.57 g) were placed in a 100 mL round bottom flask, and K 2 CO 3 (30 mmol, 4.15 g) and 50 mL of acetone, reflux, TLC to track the progress of the reaction, after the reaction is complete, filter, wash with acetone, spin dry, and purify by column chromatography to obtain a yellow solid with a yield of 87%.
[0022] (2) Preparation of o-methylazidobenzene
[0023]
[0024] Add a mixed solution of concentrated hydrochloric acid and water (14 mL, 1:1, v / v) into a 250 mL two-necked flask, add o-toluidine (22 mmol, 2.36 g), and stir until dissolved. At 0-5 °C, 8 mL of sodium nitrite (22 mmol, 1.52 g) in ice-water solution was slowly added dropwise. After 20 minutes, slowly add 18 mL of dissolved NaN 3 (22 mmol, 1.43 g) in water. After the dropwise addition, stir at room temperature, follow the reaction by thin...
Embodiment 2
[0030] 5-Hydroxy-2-phenyl-7-((1-(o-tolyl)-1 H -1,2,3-Triazol-4-yl)methoxy)-4 H - Preparation of chroman-4-one
[0031]
[0032]Add 5-hydroxy-2-phenyl-7-(propyl-2-ynyl-1-yloxy)-4 H - Chroman-4-one (0.68 mmol, 200 mg), o-methylazidobenzene (0.82 mmol, 109 mg) and water (3 mL). Then add catalyst cuprous sulfide (0.34 mmol), ultrasonic reaction at room temperature, TLC monitoring. After the reaction was complete, it was extracted with dichloromethane, washed with water, dried over anhydrous sodium sulfate, filtered with suction, precipitated, and subjected to column chromatography to obtain a light yellow solid with a yield of 85%.
Embodiment 3
[0034] 7-((1-(4-bromophenyl)-1 H -1,2,3-Triazol-4-yl)methoxy)-5-hydroxyl-2-phenyl-4 H - Preparation of chroman-4-one
[0035]
[0036] Add 5-hydroxy-2-phenyl-7-(propyl-2-ynyl-1-yloxy)-4 H - Chroman-4-one (0.68 mmol, 200 mg), p-bromoazidobenzene (0.68 mmol, 135 mg) and water (3 mL). Then add catalyst cuprous sulfide (0.34 mmol), ultrasonic reaction at room temperature, TLC monitoring. After the reaction was complete, it was extracted with dichloromethane, washed with water, dried over anhydrous sodium sulfate, filtered with suction, precipitated, and subjected to column chromatography to obtain a light yellow solid with a yield of 88%.
[0037] 1 H NMR (400 MHz, [D 6 ]DMSO): δ = 12.84 (s, 1H, OH), 9.04 (s, 1H, CH-H), 8.11 (d, J = 5.8 Hz, 2H, Ar-H), 7.91 (d, J = 8.1 Hz, 2H, Ar-H), 7.82 (d, J = 8.1 Hz, 2H, Ar-H), 7.65-7.59 (m, 3H, Ar-H), 7.06 (s, 1H, CH-H), 7.01 (s, 1H, Ar-H), 6.55 (s, 1H, Ar-H), 5.41 (s, 2H, CH 2 -H). ESI MS m / z : 490 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com