Kit for detecting chlamydia trachomatis (CT)
A detection kit and technology for Chlamydia trachomatis, which are applied in the direction of determination/inspection of microorganisms, fluorescence/phosphorescence, biochemical equipment and methods, etc., can solve the problem of low detection sensitivity, and achieve high detection sensitivity, wide detection range and fast operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
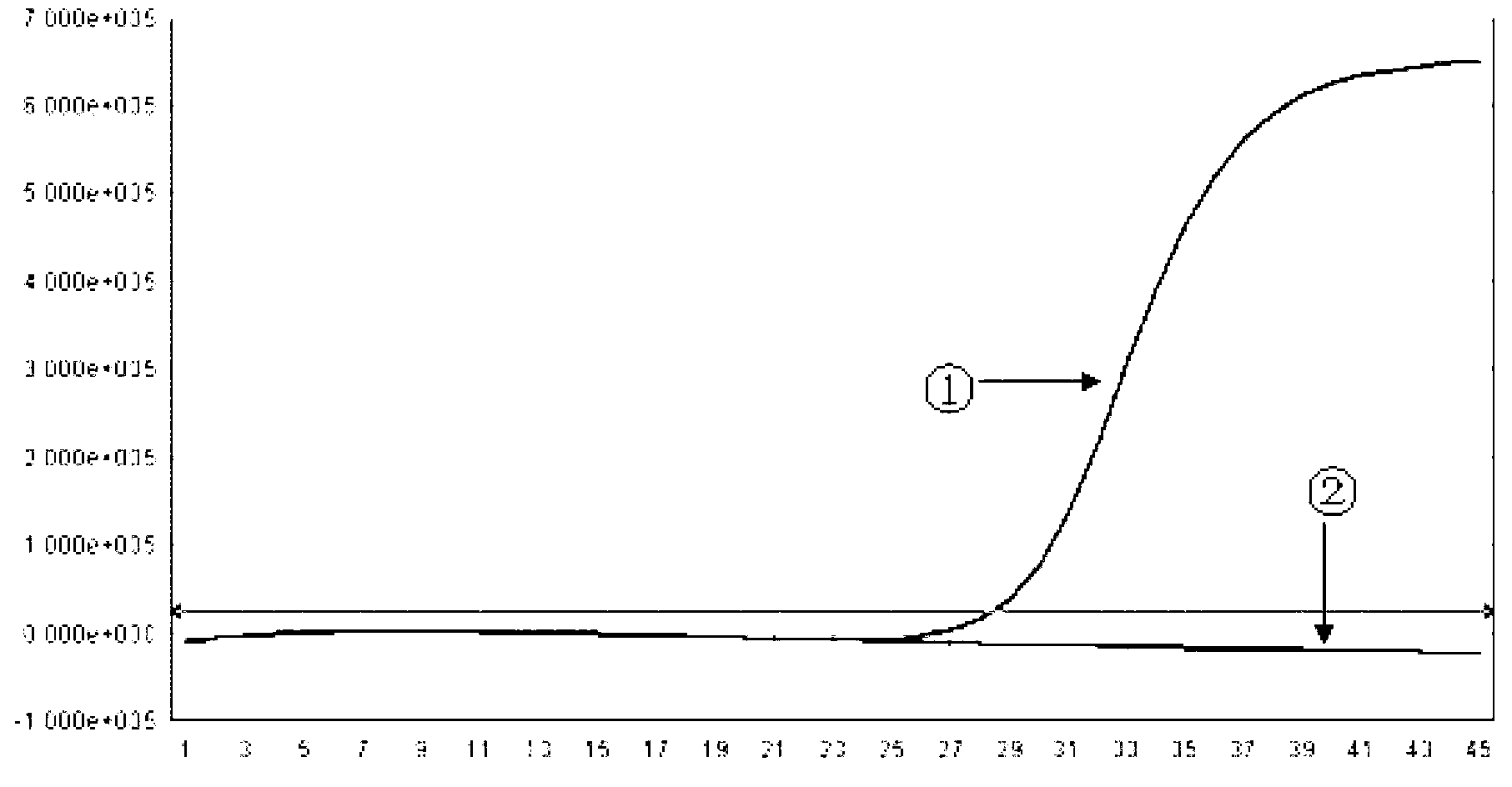
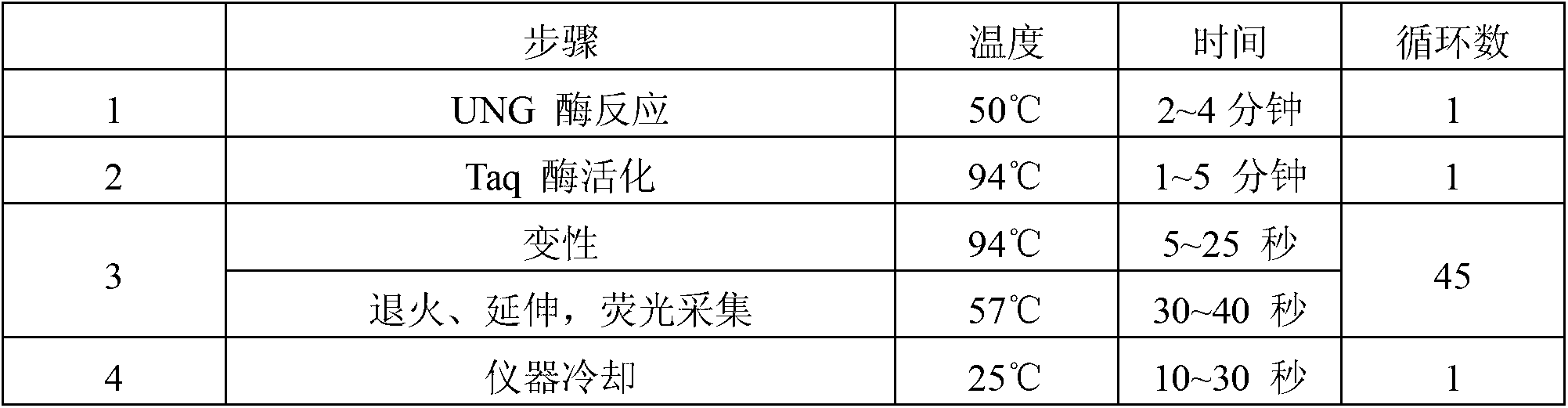
Image
Examples
Embodiment 1
[0026] The present embodiment provides a specific Chlamydia trachomatis fluorescent PCR detection kit, which includes the following components:
[0027] ①Nucleic acid release agent: contains 0.1mM / L of surfactin, 100mM / L of potassium chloride, 0.1% of sodium dodecylsulfonate (SDS), and 0.1% of ethanol.
[0028] ②Internal standard (positive internal control): It is a recombinant of a 97 base pair artificially synthesized DNA sequence inserted into the pUC18T vector, that is, a plasmid, the concentration is 5.00E+05copies / ml, and the sequence of 97 base pairs is: 5'-GTGTCTGCGGCGTTTTATCATCATCTTCCTCTGTCATCCAGTGCAAGTCTTGATCCTGTCGTTGGTTCTTCTGGACTATCAAGGTATGTTGCCCGTTTGT-3'.
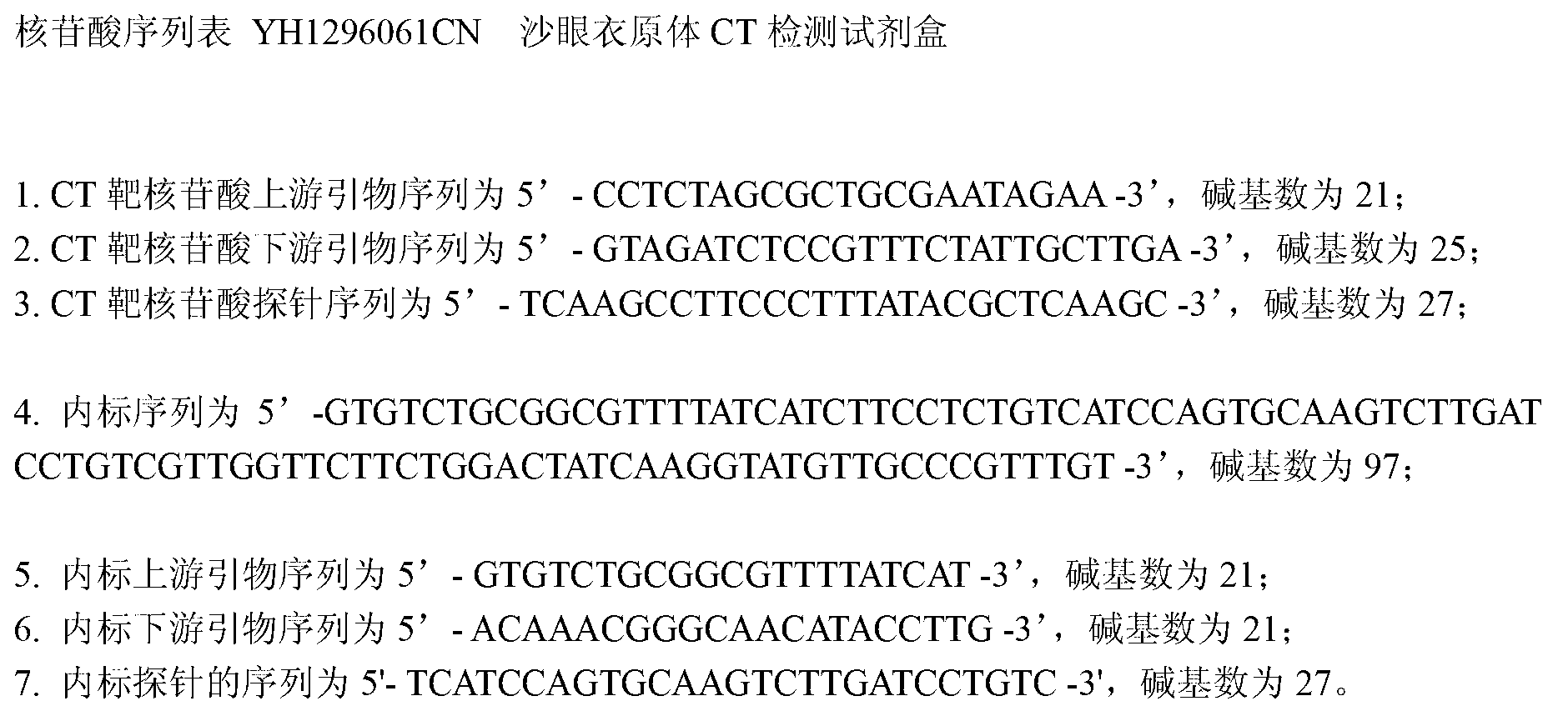
[0029] ③PCR reaction solution: including 5 μl of 10×PCR reaction buffer, 0.2 mmol / L dNTP, 0.3 μmol / L upstream and downstream primers for target polynucleotide amplification, and 0.3 μmol / L probe for target polynucleotide detection The upstream and downstream primers used for internal standard fragment amplifica...
Embodiment 2
[0034] This embodiment provides the operation steps of the kit described in the above-mentioned embodiment 1 for detecting CT-DNA in unknown samples such as genital secretions:
[0035] 1. Reagent preparation
[0036] According to the number of samples to be tested, CT negative control and CT positive control, take the corresponding amount of PCR reaction solution (38 μl / person), enzyme mixture (2 μl / person) and internal standard 1.0 μl / person in proportion, fully Mix well to form a PCR-mix. For example, when the samples to be tested are for 3 people, a total of 5 people should be prepared (the number of people for the above three is 3, 1, and 1 respectively) PCR-mix; centrifuge briefly and set aside.
[0037] 2. Sample processing
[0038] 1. Method A: Rapid nucleic acid release directly from the sample
[0039] Add 2-5 μl of nucleic acid release agent to each PCR reaction tube (deep aspiration and shallow pipetting are recommended to avoid air bubbles), add 3-5 μl each of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com