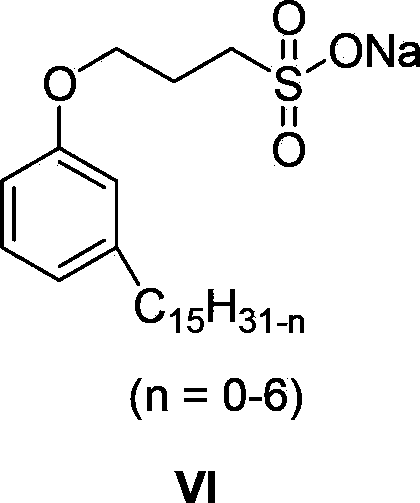
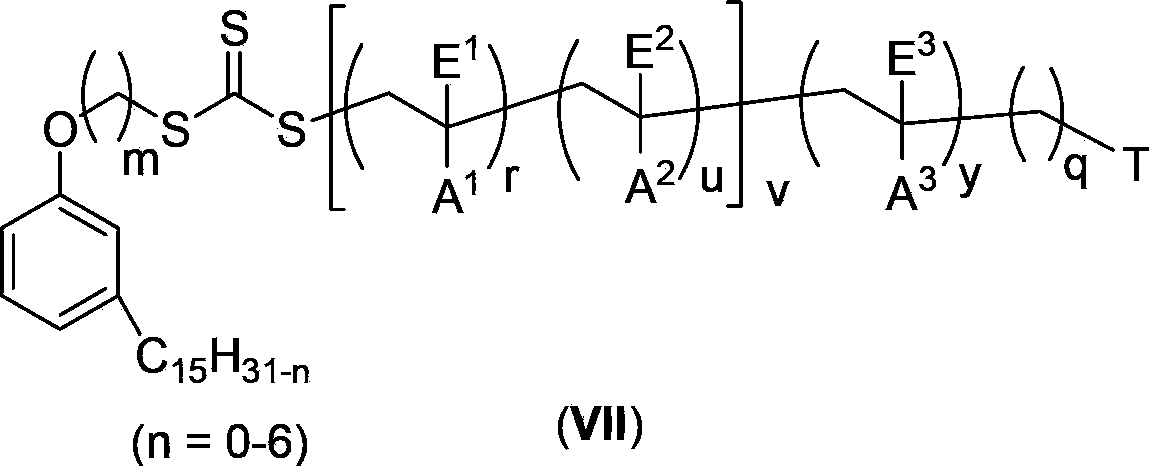
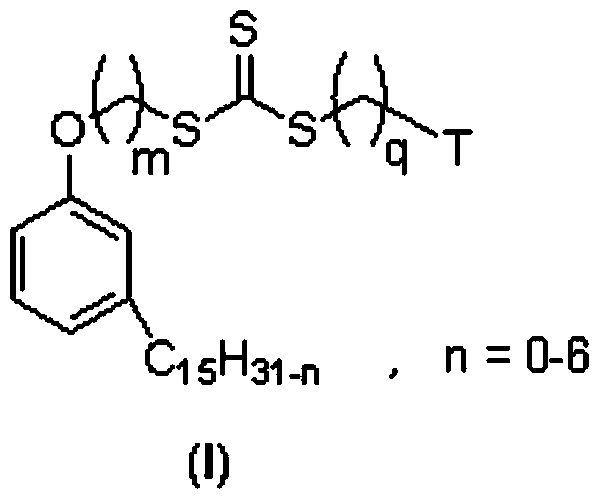
Cardanol-based trithiocarbonate and its synthesis method and application
A technology of thiol compounds and carboxyl groups, applied in the field of chemistry, can solve the problems of inability to use coatings, inks, adhesives, polymers, poor water resistance, poor scratch resistance, etc., and achieves a wide range of applicable monomers and broad application prospects. , the effect of a simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 4-bromobutyl cardanol ether (IVa)
[0027] The target compound was prepared from cardanol, 1,4-dibromobutane and alkali.
[0028] 1,4-Dibromobutane (2.70g, 12.5mmol), sodium bicarbonate (8.4g, 100mmol) and tetrahydrofuran (5mL) were added to the reaction flask, then cardanol (1.21g, 4mmol) was slowly added dropwise into the reaction mixture. After adding the material, react at room temperature for 24h. Add 100mL of water to the system, separate the layers, add anhydrous sodium sulfate to the organic phase to dry, and finally evaporate the solvent to obtain a yellow oil. The crude product was purified by silica gel column (eluent: ether / dichloromethane=1 / 1, volume ratio) to obtain pure product (1.25g, 71%).
[0029] 1 H NMR (400MHz, CDCl 3 ):δ7.24-7.15(m,1H),6.83-6.71(m,3H),5.89-5.82(m,0.3H),5.49-5.34(m,3H),5.12-5.00(m,0.6H) ,4.02(t,2H,J=6.0Hz),3.54-3.44(m,5H),2.88-2.81(m,2H),2.63(m,2H),2.16-2.00(m,8H),1.99-1.92 (2H),1.66-1.57(m,2H)...
Embodiment 2
[0031] Example 2 Preparation of Cardanol-based Reversible Addition-Fragmentation Chain Transfer Reagent (Ia) Using Compound IVa Synthesized in Example 1
[0032] The target compound was prepared from compound IVa, carbon disulfide, 3-mercaptopropionic acid and base.
[0033] Sodium carbonate (0.74g, 7mmol), 3-mercaptopropionic acid (0.75g, 7mmol), and acetone (30mL) were added to the reaction flask, the mixture was stirred at reflux temperature for 30min, cooled, 2mL of carbon disulfide was added, and then the mixture was Stir at room temperature for 1 h. Compound IVa (3.0g, 7mmol) was slowly added into the reaction system, and then reacted at room temperature for 2h. After the reaction was completed, it was concentrated under reduced pressure to obtain a yellow oil. The oil was dissolved in dichloromethane, washed 5 times with water, dried over anhydrous sodium sulfate, filtered and concentrated to obtain a crude product. The crude product was purified by silica gel column...
Embodiment 3
[0037] Example 3 Reversible addition-fragmentation chain transfer polymerization of methyl methacrylate (MMA) using the cardanol-based reversible addition-fragmentation chain transfer reagent (Ia) prepared in Example 2
[0038] Methyl methacrylate (3.00 g, 30 mmol), cardanol group reversible addition-fragmentation chain transfer reagent (Ia) (0.54 g, 1.0 mmol), potassium persulfate (0.13 g, 0.57 mmol) were dissolved in acetonitrile (25 mL )middle. Under ice-water cooling, the reaction flask was first evacuated, then filled with nitrogen, and repeated three times. The reaction solution was heated to 70° C. and kept constant, and then sodium sulfite (0.15 g, 1.25 mmol) was added for reaction. After 4 hours, the heating was stopped, and water or lower alcohols such as methanol and ethanol were added to the reaction flask, and a precipitate was generated, and the solid was filtered out, and the solid was dried in a vacuum oven at 65° C. for 24 h. Monomer conversion was determine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com