Method for modifying surface of anode material for lithium ion battery
A lithium-ion battery and positive electrode material technology, applied in the field of lithium-ion batteries and electrochemistry, can solve the problems of low initial efficiency, poor rate performance and cycle stability, etc., achieve convenient operation, improve initial charge and discharge efficiency, and have obvious effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
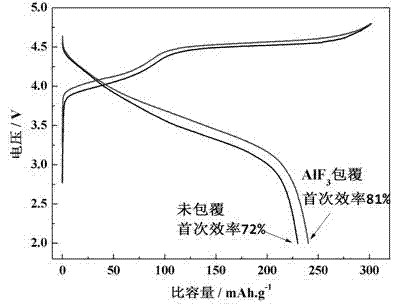
[0023] Weigh 0.1786g of Al(NO 3 ) 3 9H 2 O and 0.0529 g of NH 4 F, add 10ml deionized water respectively, stir for 1h to dissolve completely. Utilize the NaOH solution that mass concentration is 5% to adjust pH of aluminum nitrate solution to be 8, then weigh 2.5g Li 1.2 Ni 0.13 co 0.13 mn 0.54 o 2 , was added to the aluminum nitrate solution, stirred for 0.5h to obtain a uniform mixture, and then the ammonium fluoride solution was slowly added dropwise to the above mixture, and continued to stir for 10h. The product was collected, then suction filtered and washed three times, and the sample was placed in a vacuum drying oven at 80°C overnight. Finally, the material was sintered in high-purity argon at 400 degrees for 10 hours to obtain AlF 3 Surface coated with modified Li 1.2 Ni 0.13 co 0.13 mn 0.54 o 2 .
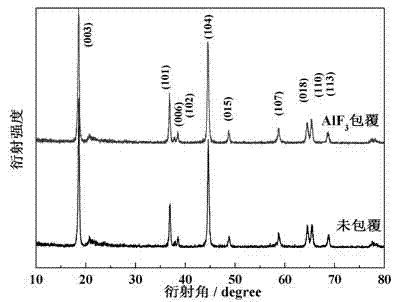
[0024] The X-ray diffraction patterns of materials before and after coating are shown in figure 1 As shown, it can be seen from the figure that AlF 3 coa...
Embodiment 2
[0026] Adopt the same method as embodiment 1 to prepare AlF 3 Surface-modified cathode materials, the difference is the synthesis of AlF 3 The material is Al 2 (SO 4 ) 3 and NaF, the alkali solution to adjust the pH value is ammonia water, the final pH is 6, and the positive electrode material added is Li 1.19 Ni 0.16 co 0.08 mn 0.57 o 2 , using freeze-drying, and finally sintering at 700°C for 1h in high-purity nitrogen.
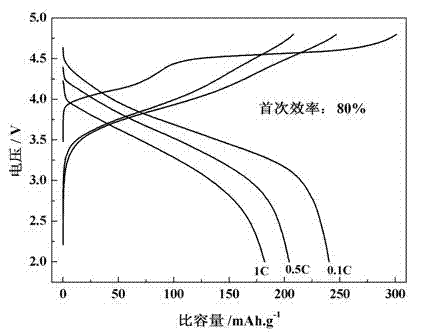
[0027] After the obtained coating material is assembled into a battery, the first charge and discharge curves at different rates are shown in image 3As shown, the first discharge specific capacities at 0.1C, 0.5C and 1C rates are 240.3, 204.2 and 182 mAh / g, respectively. Figure 4 is the cycle performance graph, the capacity retention rates of the materials before and after coating are 93.95% and 97.56% after 50 cycles at 1C rate.
Embodiment 3
[0029] Adopt the same method as embodiment 1 to prepare AlF 3 Surface-modified cathode materials, the difference is the synthesis of AlF 3 The material is Al(NO 3 ) 3 and KF, the alkali solution to adjust the pH value is potassium hydroxide, the final pH is 11, and the anode material added is LiNi 1 / 3 co 1 / 3 mn 1 / 3 o 2 , Stirred for 2h to collect the product. The sample was not washed, and the drying method was stirring and drying in a water bath, and finally sintered at 500 ° C for 8 h in high-purity helium.
[0030] Figure 5 is a scanning electron micrograph of the material before and after coating, where a is the sample before coating, and b is the sample after coating. It can be seen from the figure that the surface of the material after coating modification becomes denser and smoother, which is due to the coating of a layer of AlF on the surface of the material. 3 due to.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com