Applications of tea double-chlorine carboxamide and tea double-bromine carboxamide or like in preparation of products for prevention and treatment of diseases such as cancer
A technology of tea bisbromide and tea bischloramide, which is applied in the medical field, can solve problems such as restriction prevention and side effects, and achieve the effects of good curative effect, small side effects, and reduced side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
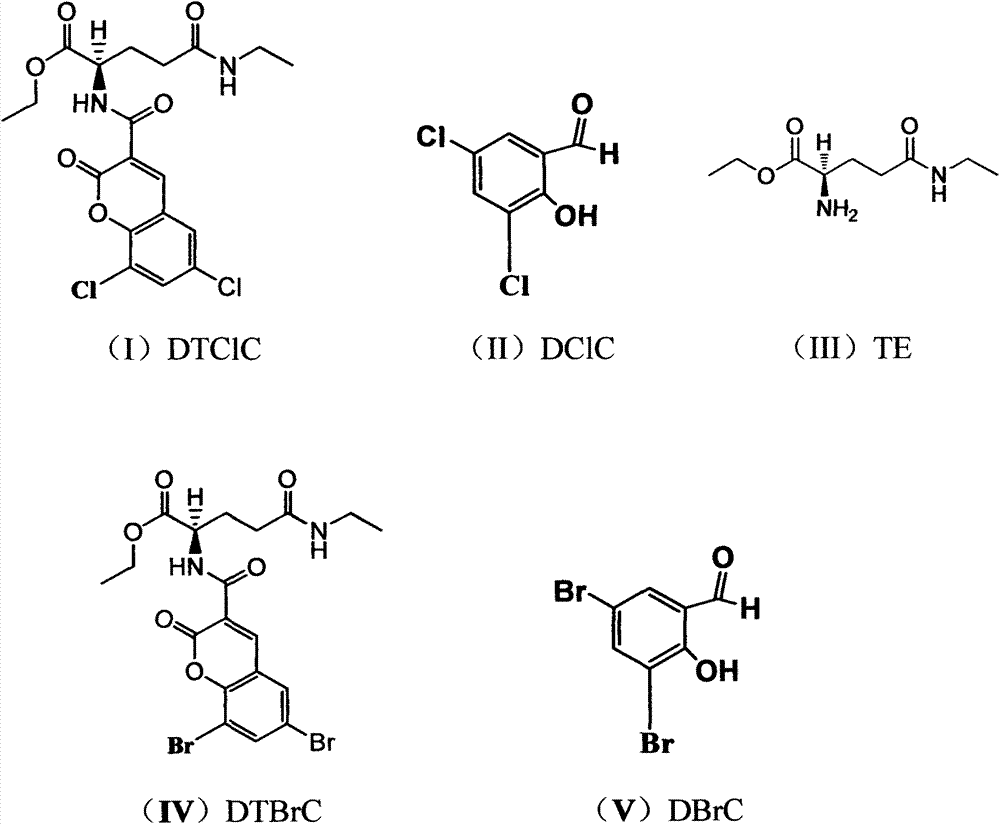
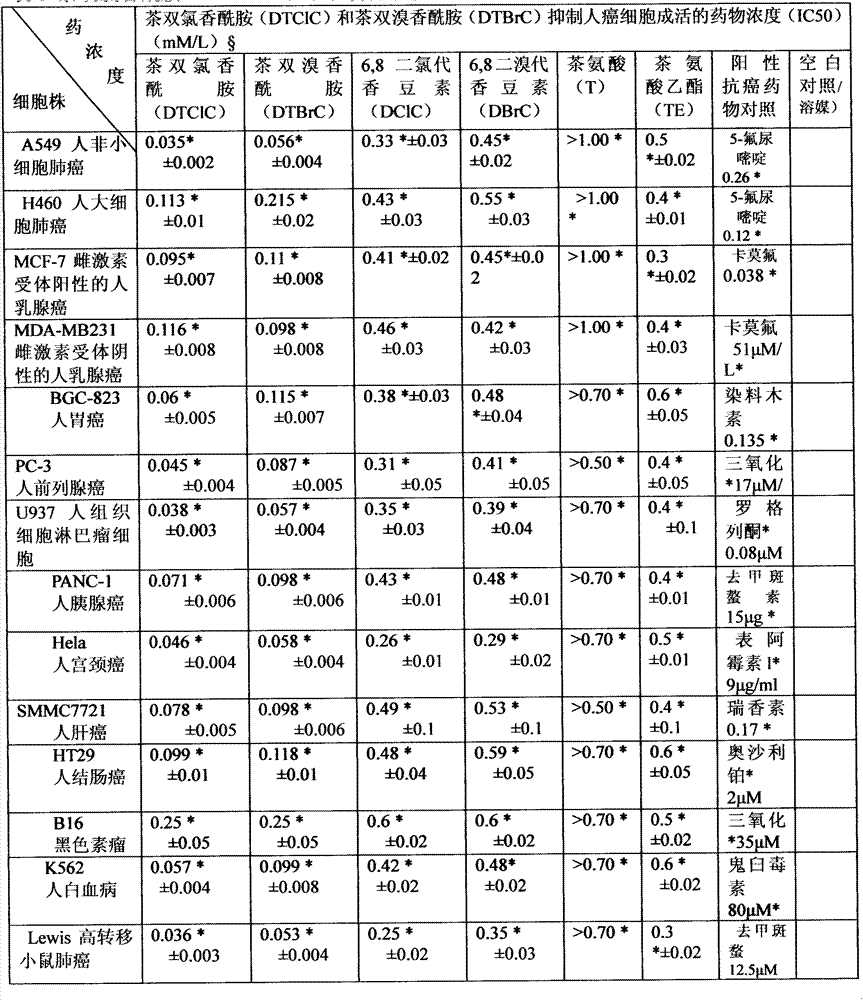
[0041] Two compounds for the treatment of tumors in this example, the name of which is (R)-ethyl ester-2-(6,8-dichloro-2-oxo-2H-benzopyran-3-carboxamide) -5-(Ethylamino)-5-oxopentanoic acid ester, referred to as tea dichloroaromatic amide (DTClC), has a chemical structural formula shown in formula (I); its 2 names are (R)-ethyl ester-2- (6,8-dibromo-2-oxo-2H-benzopyran-3-carboxamido)-5-(ethylamino)-5-oxopentanoate, referred to as tea bisbromamide (DTBrC ), having a chemical structural formula shown in formula (IV);
[0042] As the compound shown in formula (I) and formula (IV), the physical properties are all pale yellow powdery solids, and the melting point decomposes above 136° and 121° Celsius respectively;
[0043] The compound shown in formula (I) and formula (IV) is a pharmaceutically acceptable carrier; the pharmaceutically acceptable carrier includes any substance suitable for injection, preferably water for injection, or emulsifying agent, lipid body, nano preparati...
Embodiment 2
[0058] The difference between this embodiment and embodiment 1 is:
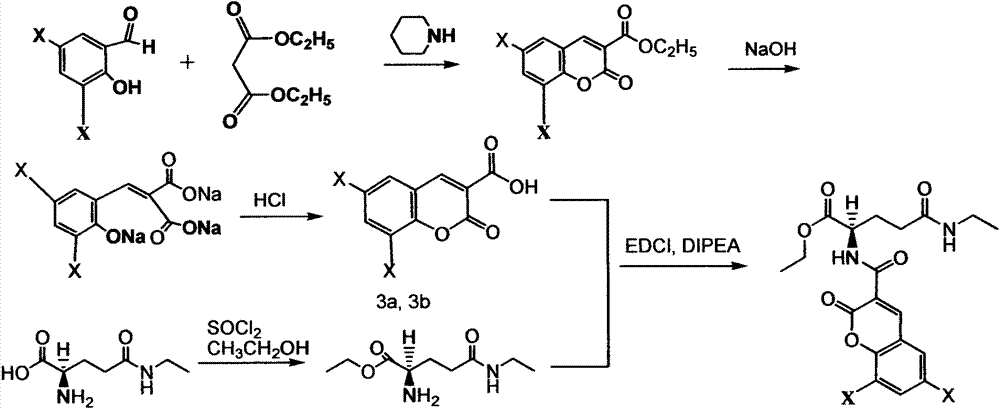
[0059] Step 1: Preparation of 6,8-dichlorocoumarin-3-carboxylic acid and 6,8-dibromocoumarin-3-carboxylic acid
[0060] (1) Add 205g of 3,5-dichlorosalicylaldehyde or 3,5-dibromosalicylaldehyde, 205mL of diethyl malonate, 610mL of absolute ethanol, 11mL of hexahydropyridine and 1-1.3ml of glacial acetic acid; (2) Under anhydrous conditions, in a water bath at 80°C, stir and reflux for 2.5 hours, then cool; (3) Add about 610mL of cold water (0°C), filter with suction after crystallization, and cool with 110mL of ice water (0°C) Wash twice with 50% ethanol to get 6,8-dichlorocoumarin-3-acidate or 6,8-dibromocoumarin-3-acidate; (4) add 6 , 8-dichlorocoumarin-3-condensate or 6,8-dibromocoumarin-3-condensate 128g and 103g sodium hydroxide, then add 510ml of absolute ethanol and 510mL of water, 80 ℃ water bath Under certain conditions, heat to reflux for about 2.5 hours; (5) After the reaction is completed, immed...
Embodiment 3
[0066] The difference between this embodiment and embodiment 2 is that
[0067] Step 1: Preparation of 6,8-dichlorocoumarin-3-carboxylic acid or 6,8-dibromocoumarin-3-carboxylic acid
[0068] (1) Add 210g of 3,5-dichlorosalicylaldehyde or 3,5-dibromosalicylaldehyde, 210mL of diethyl malonate, 615mL of absolute ethanol, 12mL of hexahydropyridine and 1-1.3ml of glacial acetic acid; (2) Under anhydrous conditions, stir and reflux for 3 hours in a water bath at 80°C, and cool; (3) Add about 615mL of cold water (0°C), filter with suction after crystallization, and cool with 115mL of ice water (0°C) Wash twice with 50% ethanol to obtain 6,8-dichlorocoumarin-3-acidate or 6,8-dibromocoumarin-3-acidate; (4) add 6, 132g of 8-dichlorocoumarin-3-condensate or 6,8-dibromocoumarin-3-condensate and 106g of sodium hydroxide, then add 515ml of absolute ethanol and 515mL of water, in a water bath at 80°C (5) Immediately after the reaction is completed, put it in an ice bath at 0°C, add concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com