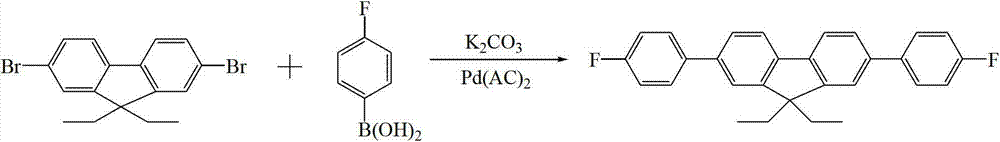Fluorobenzofiurene organic fluorescent material and preparation method thereof
A fluorescent material, the technology of fluorophenylfluorene, which is applied in the field of fluorophenylfluorene organic fluorescent materials and its preparation, can solve the problems of limiting the application of fluorene organic fluorescent materials, harsh preparation conditions, cumbersome synthesis steps, etc., and achieve good photoelectric activity, Simple preparation method and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
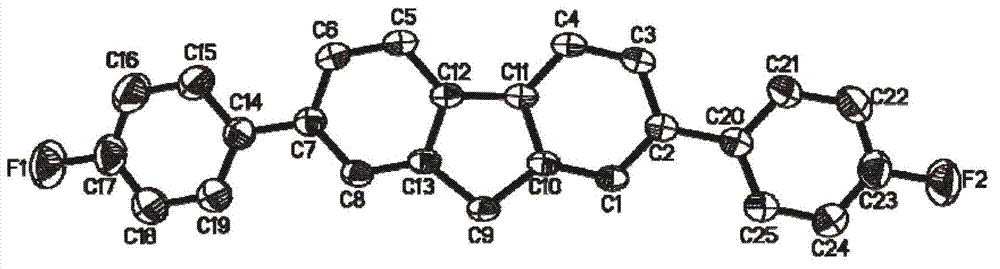
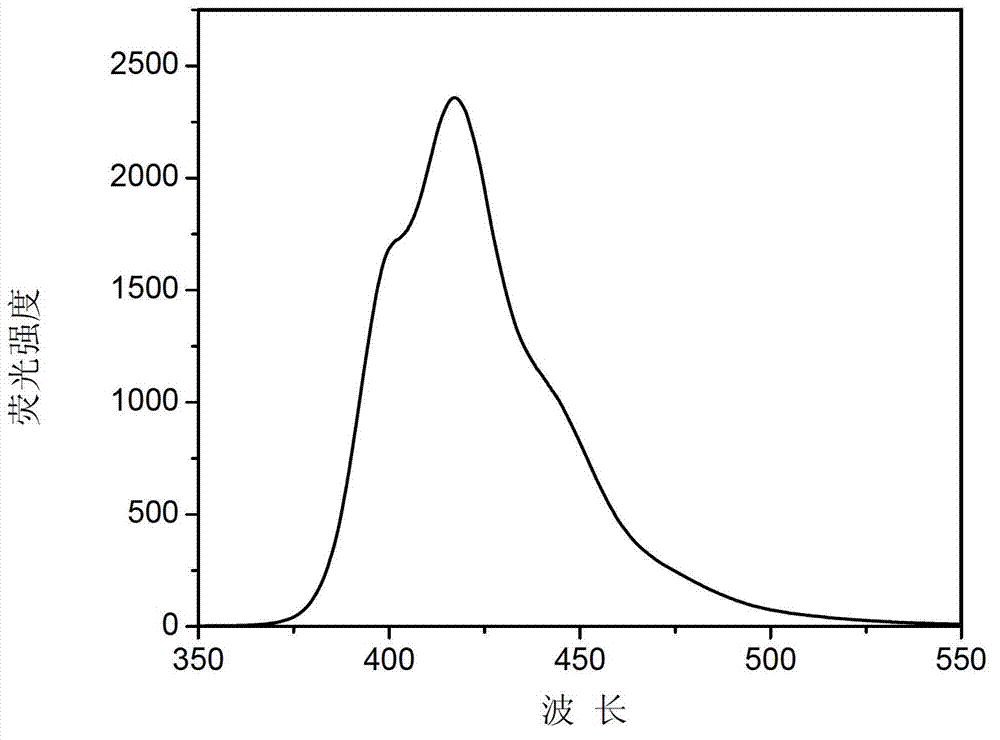
[0020] Take 2,7-dibromofluorene (0.33g, 1.0mmol), 4-fluorophenylboronic acid (0.35g, 2.5mmol), palladium acetate (0.012g, 0.05mmol) and potassium carbonate (0.70g, 5mmol) as solid powder ) were mixed in a 100ml three-necked flask; then a mixed solution prepared from 30mL isopropanol (i-PrOH) and 6mL water was injected into the three-necked flask, and the reaction was refluxed at 80°C for 1 day in an air atmosphere; the reaction After the reaction solution is cooled, extract with dichloromethane, combine the organic phases, then wash the organic phase with saturated brine, then dry with anhydrous magnesium sulfate for 12 hours, and obtain the filtrate after filtration; Chloromethane, to obtain a solid crude product, the mixed solvent of petroleum ether and dichloromethane with a volume ratio of 6:1 is used as an eluent, and the crude product is separated and purified by column chromatography, and finally petroleum ether and dichloromethane are removed A white powdery solid was ...
Embodiment 2
[0022] Take 2,7-dibromofluorene (0.33g, 1.0mmol), 4-fluorophenylboronic acid (0.35g, 2.5mmol), palladium acetate (0.012g, 0.05mmol) and potassium carbonate (0.70g, 5mmol) as solid powder ) were mixed in a 100ml three-necked flask; then, a mixed solution prepared by 30mL isopropanol (i-PrOH) and 6mL water was injected into the three-necked flask, and the reaction was refluxed at 90°C in an air atmosphere for 1 day; the reaction After the reaction solution is cooled, extract with dichloromethane, combine the organic phases, then wash the organic phase with saturated brine, then dry with anhydrous magnesium sulfate for 10 h, and obtain the filtrate after filtration; spin dry the isopropanol and di Chloromethane, to obtain a solid crude product, the mixed solvent of petroleum ether and dichloromethane with a volume ratio of 5:1 is used as an eluent, and the crude product is subjected to column chromatography separation and purification, and finally petroleum ether and dichlorometha...
Embodiment 3
[0024] Take 2,7-dibromofluorene (0.66g, 2.0mmol), 4-fluorophenylboronic acid (0.70g, 5mmol), palladium acetate (0.024g, 0.10mmol) and potassium carbonate (1.40g, 10mmol) as solid powder Mix in a 150ml three-necked flask; then inject a mixed solution prepared from 60mL isopropanol (i-PrOH) and 15mL water into the three-necked flask, and reflux at 80°C for 1 day in an air atmosphere; the reaction is complete After the reaction solution is cooled, extract with dichloromethane, combine the organic phases, then wash the organic phase with saturated brine, then dry with anhydrous magnesium sulfate for 12 hours, and obtain the filtrate after filtration; spin dry the isopropanol and dichloromethane in the filtrate Methane, to obtain solid crude product, the mixed solvent that petroleum ether and dichloromethane are formed with the volume ratio of 6:1 is used as eluting agent, carries out column chromatography separation and purification to crude product, finally removes petroleum ether...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com