Polymeric photoinitiator and preparation method thereof
A technology for polymerizing photoinitiators and hydroxyl groups, which is applied in the field of photosensitive polymer materials, and can solve problems such as affecting material properties, polluting food, and reducing reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
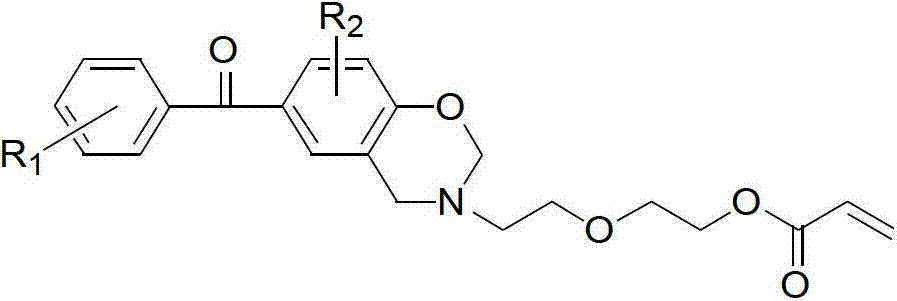
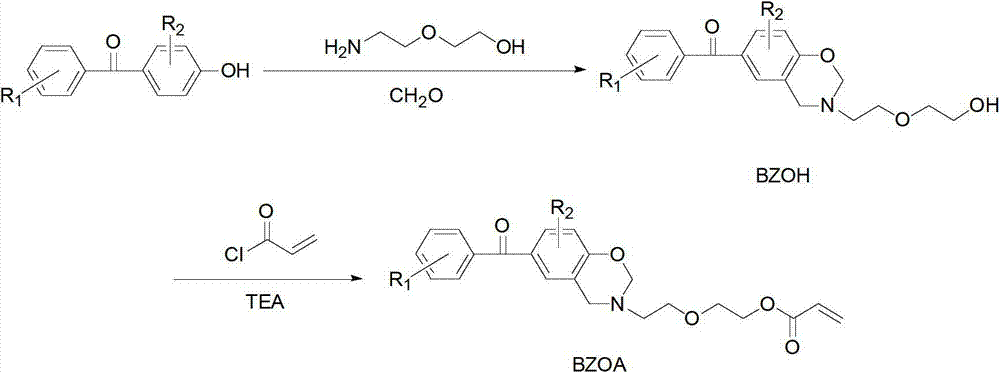
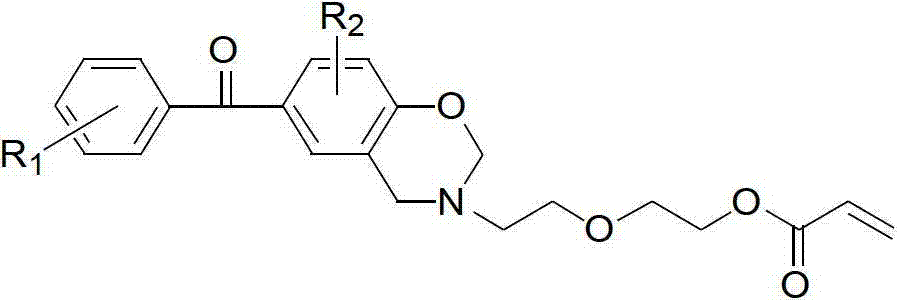
[0023] (a) Dissolve 1.2 parts of p-hydroxybenzophenone, 2 parts of paraformaldehyde, and 1 part of diglycolamine in 30 parts of 1,4-dioxane, and stir until completely dissolved. Slowly heat to reflux at a heating rate of 5°C / min, keep the reflux reaction for 5 hours, stop heating and cool to room temperature, distill off the solvent under reduced pressure, add chloroform to dissolve, wash 5 times with 0.1N sodium hydroxide aqueous solution, deionize Wash with water 5 times. Then the organic layer was dried with anhydrous sodium sulfate, filtered, the filtrate was evaporated to remove the solvent, and dried in vacuo to obtain an orange-red oily liquid (BZOH).
[0024] (b) Dissolve 1 part of BZOH and 1.2 parts of triethylamine (TEA) in 30 parts of dichloromethane, add 1.2 parts of acryloyl chloride into the constant pressure dropping funnel, and control the dropping speed to 4-5s / drop, Ice bath, avoid light, after magnetic stirring for 24h, filter, add saturated sodium bicarbon...
Embodiment 2
[0026] (a) Dissolve 1.4 parts of p-hydroxybenzophenone, 2 parts of paraformaldehyde, and 1 part of diglycolamine in 30 parts of butanone, and mechanically stir until completely dissolved. Slowly heat to reflux at a heating rate of 5°C / min, keep the reflux reaction for 10 hours, stop heating and cool to room temperature, distill off the solvent under reduced pressure, add chloroform to dissolve, wash 5 times with 0.1N sodium hydroxide aqueous solution, deionize Wash with water 5 times. Then the organic layer was dried with anhydrous sodium sulfate, filtered, the filtrate was evaporated to remove the solvent, and dried in vacuo to obtain an orange-red oily liquid (BZOH).
[0027] (b) Dissolve 1 part of BZOH and 1.5 parts of triethylamine (TEA) in 30 parts of dichloromethane, add 1.5 parts of acryloyl chloride into the constant pressure dropping funnel, and control the dropping speed to 4-5s / drop, Ice bath, protected from light, after magnetic stirring for 18h, filter, add satur...
Embodiment 3
[0029](a) Dissolve 1.2 parts of 4,4'-dihydroxybenzophenone, 2 parts of paraformaldehyde, and 1 part of diglycolamine in 30 parts of 1,4-dioxane, and stir until complete dissolve. Slowly heat to reflux at a heating rate of 5°C / min, keep the reflux reaction for 5 hours, stop heating and cool to room temperature, distill off the solvent under reduced pressure, add chloroform to dissolve, wash 5 times with 0.1N sodium hydroxide aqueous solution, deionize Wash with water 5 times. Then the organic layer was dried with anhydrous sodium sulfate, filtered, the filtrate was evaporated to remove the solvent, and dried in vacuo to obtain an orange-red oily liquid (BZOH).
[0030] (b) Dissolve 1 part of BZOH and 1.5 parts of triethylamine (TEA) in 30 parts of dichloromethane, add 1.5 parts of acryloyl chloride into the constant pressure dropping funnel, and control the dropping speed to 4-5s / drop, Ice bath, protected from light, after magnetic stirring for 12h, filter, add saturated sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com