Carbon-coated ferroferric oxide and preparation method and application of carbon-coated ferroferric oxide in lithium battery
A carbon-coated ferric tetroxide and carbon-coated technology, used in battery electrodes, secondary batteries, circuits, etc., can solve problems such as poor conductivity, loss of electrical contact, and reduced reaction reversibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
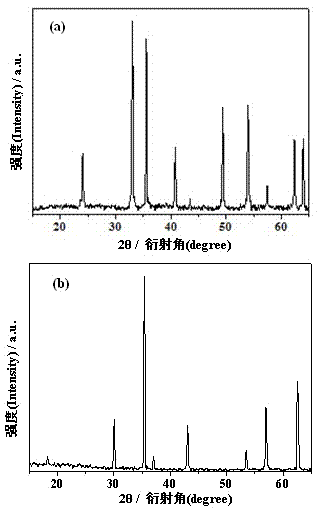
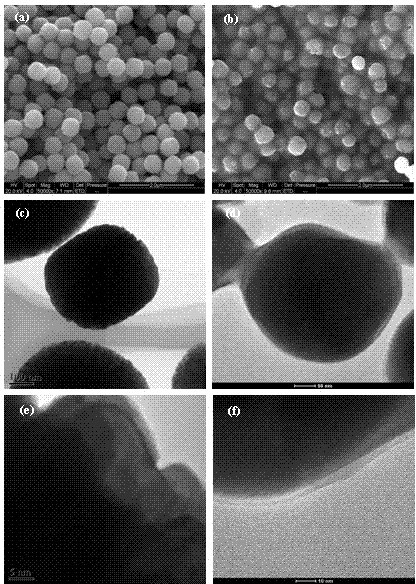
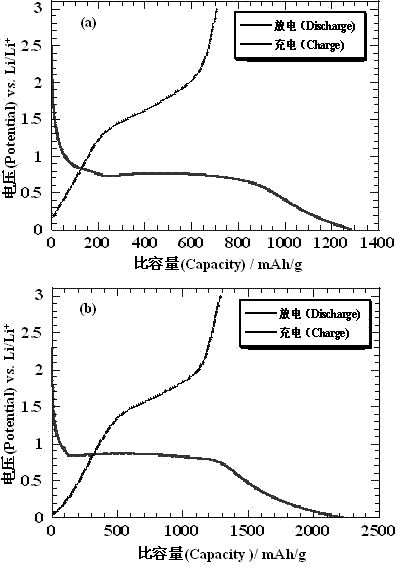
Image
Examples
Embodiment 1
[0019] Example 1 Fe 3 o 4 Preparation of C composites
[0020] (1) Add 0.40 g FeCl 3 ?6H 2 O, 0.12 g NH 4 Ac and 0.75 g PVP were dissolved in 30 ml deionized water and then transferred to a 50 ml hydrothermal reactor;
[0021] (2) Transfer the reactor to a constant temperature oven, react at 140 °C for 24 h, and then cool down to room temperature naturally;
[0022] (3) Centrifuge the product obtained in step (2) and wash with distilled water and ethanol 10 times until the supernatant is clarified;
[0023] (4) Collect the product of step (3) and dry it in a vacuum oven at 60 °C for 10 h to obtain Fe 2 o 3 red powder;
[0024] (5) Weigh 0.05 g of the product obtained in step (4) and spread it evenly in a quartz boat, put the quartz boat into the middle of a quartz tube with an inner diameter of 60 mm, and place the quartz tube in a tube furnace. The temperature was raised to 550 °C under an argon atmosphere of 100 ml / min;
[0025] (6) After the internal temperature o...
Embodiment 2
[0028] By making the CR2430 type button cell and using the LAND CT2001A type charge and discharge test system to carry out the electrochemical performance test of the sample in Example 1, the specific steps are as follows:
[0029] (1) Evenly mix the sample, conductive agent acetylene black and binder polytetrafluoroethylene emulsion according to the ratio of 50:30:20 to make a slurry;
[0030] (2) The above-mentioned slurry is rolled and compacted multiple times by a pair of rollers to obtain a sheet with a uniform thickness and a smooth surface, which is processed by punching to obtain a disc with a diameter of 14 mm;
[0031] (3) Use a tablet press (pressure of 20 MPa) to press the above disk on the copper grid of the current collector, and dry it in vacuum at 100 °C for 10 h to obtain the negative electrode sheet;
[0032] (4) In an inert atmosphere glove box, use the CR2430 button battery shell as the battery shell, stack it in the order of positive electrode (lithium met...
Embodiment 3
[0035] Example 3: Similar to Example 1, the difference is that in step (5), the temperature was raised to 600°C under an argon atmosphere, and the flow rate of acetylene was 1 ml / min. After 50 charge-discharge reactions, the final electrode material was The cycle life was stable at 970 mAh / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Current density | aaaaa | aaaaa |
| Specific volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com