Method for preparing ethylene by dehydrating ethanol under catalysis
A technology for catalytic dehydration and ethanol, applied in chemical instruments and methods, molecular sieve catalysts, and hydrocarbon production from oxygen-containing organic compounds, etc., can solve the problems of poor reaction stability, etc., to inhibit the formation of carbon deposits and reduce the rate of carbon deposits , the effect of eliminating the influence of diffusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
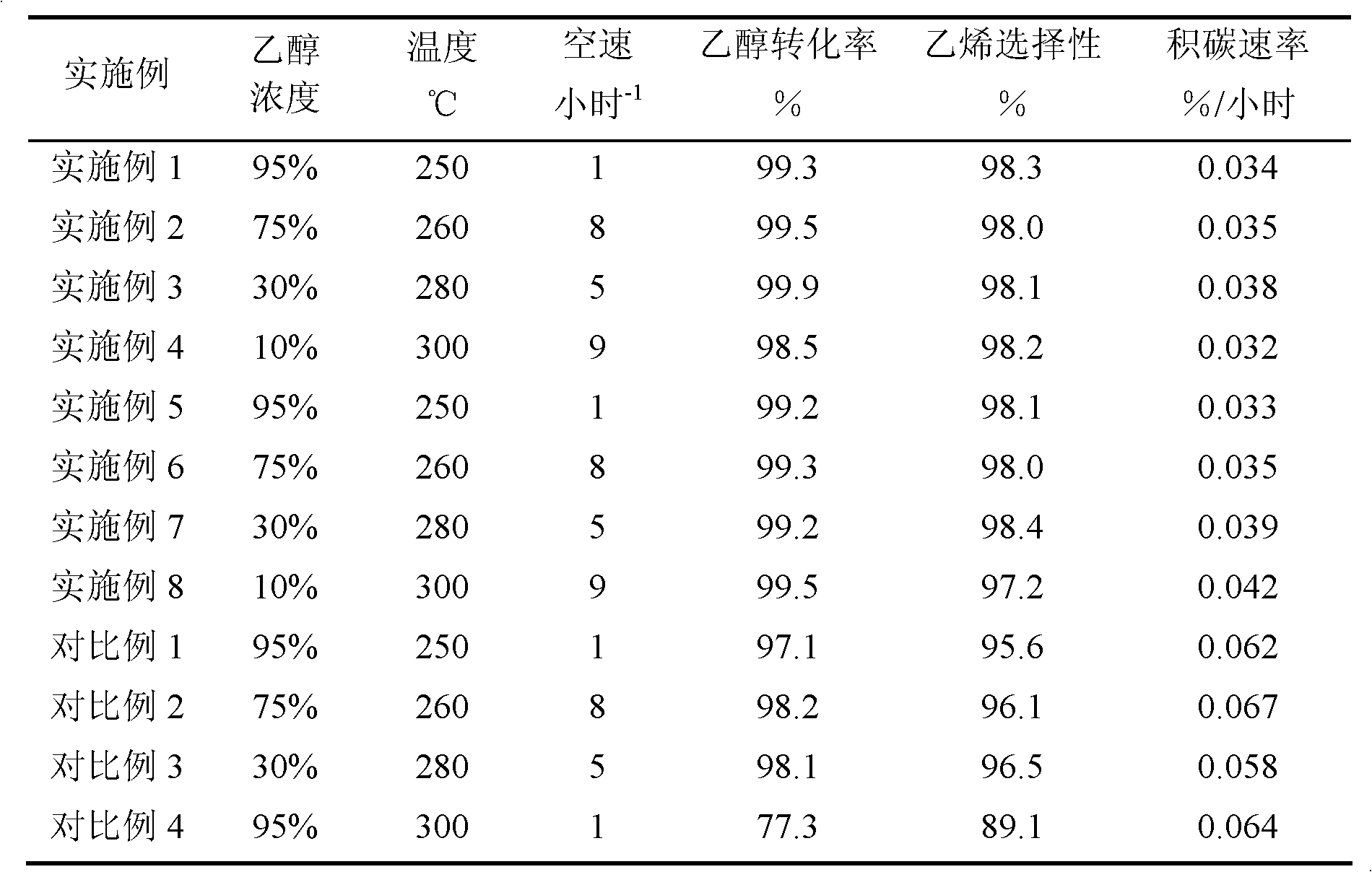
Embodiment 1
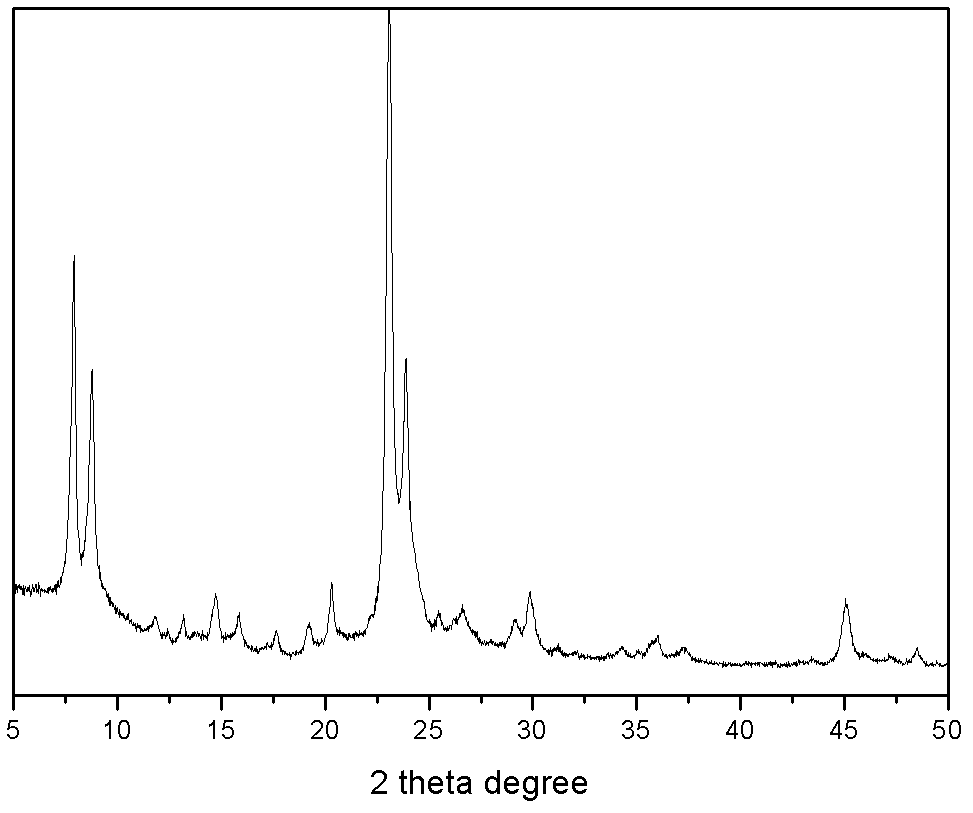
[0024] Weigh 7.4 grams of silica sol (SiO 2 Weight content 40%), add sodium metaaluminate, 40% sodium hydroxide aqueous solution again, make molar ratio be: 6.36Na 2 O:Al 2 o 3 : 80.22SiO2 2 , and add water to mix, knead and extrude. After that, it was dried at 100° C. for 1 hour, and then pelletized. A mixture of 2 grams of tetrabutylammonium bromide and 10 grams of distilled water was added in advance in the reaction kettle, a stainless steel mesh was placed above the mixture, the formed molecular sieve was placed on the stainless steel mesh, and the reaction kettle was sealed. The reactor was subjected to gas-solid phase treatment at 150°C for 10 days. After the product was taken out, it was washed with water, dried at 120°C for 10 hours, and then baked at 550°C for 5 hours to remove the template agent. For the XRD characterization results of the sample, see figure 1 . Afterwards, the obtained material was exchanged three times with 10% by weight ammonium nitrate aqueo...
Embodiment 2
[0027] Weigh 7.4 grams of silica sol (SiO 2 40% by weight), then add sodium metaaluminate, 40% aqueous sodium hydroxide solution, so that the molar ratio is: 8Na 2 O:Al 2 o 3 : 100SiO2 2 , and add water to mix, knead and extrude. Thereafter, it was dried at 120° C. for 1 hour, and then pelletized. Add a mixture of 2 grams of tetrabutylammonium hydroxide and 10 grams of distilled water in advance in the reaction kettle, place a stainless steel mesh above the mixture, place the formed molecular sieve on the stainless steel mesh, and seal the reaction kettle. The reactor was subjected to gas-solid phase treatment at 170°C for 5 days. After the product was taken out, it was washed with water, dried at 120°C for 10 hours, and then baked at 550°C for 5 hours to remove the template. Afterwards, the obtained material was exchanged three times with 10% by weight ammonium nitrate aqueous solution at 80° C., washed twice with water, dried at 120° C. for 10 hours, and calcined at 55...
Embodiment 3
[0030] Weigh 7.4 grams of silica sol (SiO 2 40% by weight), then add sodium metaaluminate, 40% aqueous sodium hydroxide solution, so that the molar ratio is: 4Na 2 O:Al 2 o 3 : 60SiO 2 , and add water to mix, knead and extrude. After that, it was dried at 120° C. for 2 hours and cut into pellets. A mixture of 7 grams of tetrabutylammonium hydroxide and 10 grams of distilled water was added to the reaction kettle in advance, a stainless steel mesh was placed above the mixture, the formed molecular sieve was placed on the stainless steel mesh, and the reaction kettle was sealed. The reactor was subjected to gas-solid phase treatment at 180°C for 3 days. After the product was taken out, it was washed with water, dried at 120°C for 10 hours, and then baked at 550°C for 5 hours to remove the template. Afterwards, the obtained material was exchanged three times with 10% by weight ammonium nitrate aqueous solution at 80° C., washed twice with water, dried at 120° C. for 10 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com