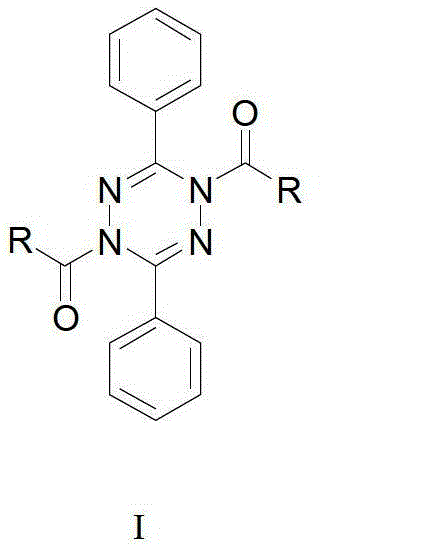
1,4-diacyl-3,6-diphenyl-1,4-dihydrotetrazine compound as well as preparation method and application thereof
A compound and diphenyl technology, applied in the field of pharmaceutical synthesis, can solve the problems of long synthetic route, inability to obtain, low yield, etc., and achieve the effects of easy operation, favorable for industrial production and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
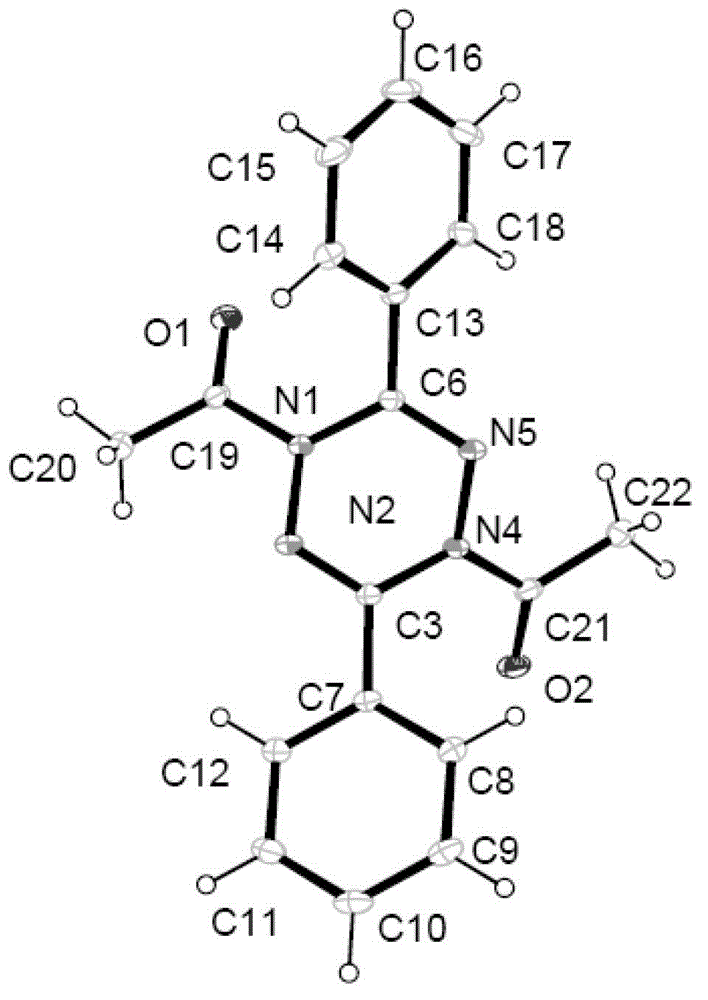
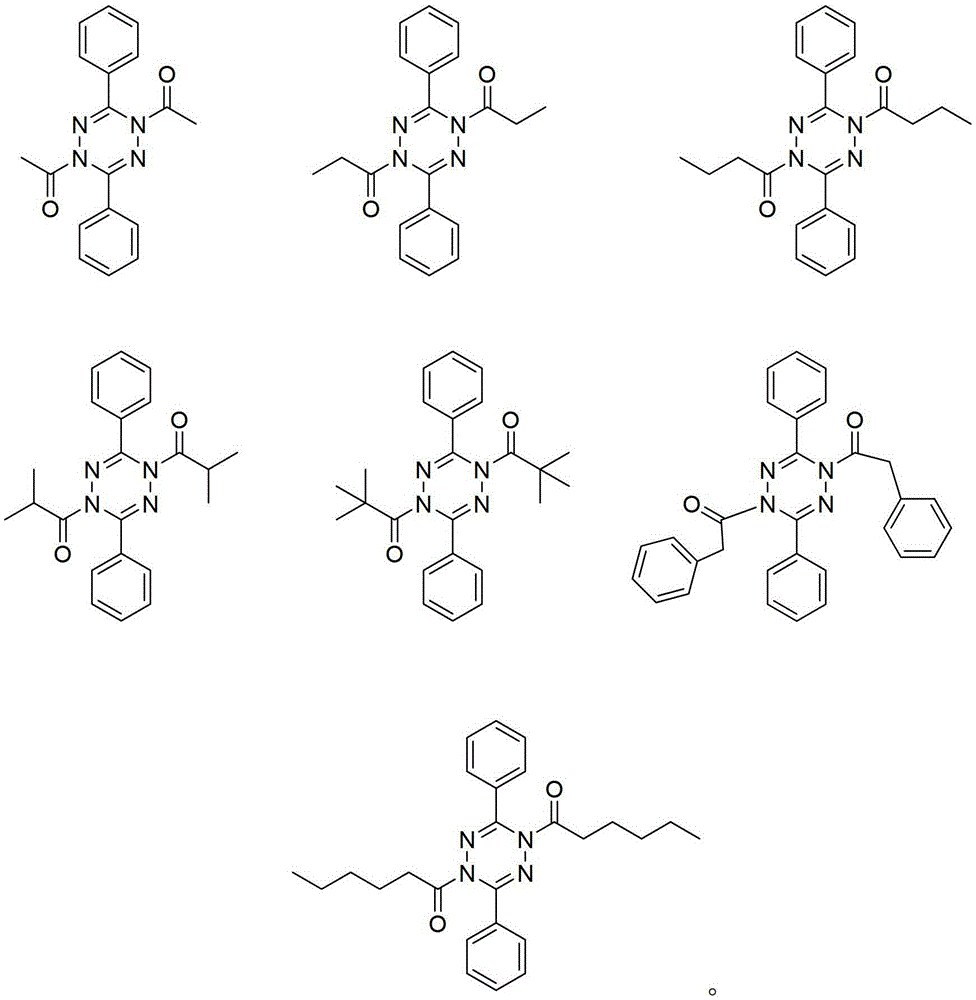
[0033] 1,4-Diacetyl-3,6-diphenyl-1,4-dihydro-s-tetrazine (Ⅰ-1)
[0034]
[0035] The preparation method of the above-mentioned 1,4-diacetyl-3,6-diphenyl-1,4-dihydro-s-tetrazine compound is as follows:
[0036] Add 0.8g (3.4mmol) of 3,6-diphenyl-1,2-dihydro-s-tetrazine and 0.85g (6.8mmol) of basic catalyst 4-(N,N-dimethylamino)pyridine into the reactor Then, add 20mL of chloroform solvent, under the condition of stirring, cool down to 0°C, then start to drop 0.53g (6.8mmol) of acetyl chloride (as a preference, first dissolve 0.53g of acetyl chloride in 10mL of chloroform solution and then dropwise), after the dropwise addition, continue to control the temperature at 0°C, and carry out the acylation reaction for 15 hours. The treatment is to remove the incompletely reacted alkaline catalyst, let it stand, separate layers, collect the organic phase, and then wash the organic phase with 30mL of 10% sodium hydroxide aqueous solution, and use alkali to remove the unreacted catal...
Embodiment 2
[0044] 1,4-dipropionyl-3,6-diphenyl-1,4-dihydro-s-tetrazine (Ⅰ-2)
[0045]
[0046] The preparation method of the above-mentioned 1,4-dipropionyl-3,6-diphenyl-1,4-dihydro-s-tetrazine compound is as follows:
[0047] Add 0.8g (3.4mmol) of 3,6-diphenyl-1,2-dihydro-s-tetrazine and 0.54g (6.8mmol) of basic catalyst pyridine into the reactor, and then add 20mL of dichloromethane solvent , under stirring conditions, lower the temperature to -5°C, then start to drop propionyl chloride 0.64g (6.97mmol) (as a preference, first dissolve 0.64g propionyl chloride in 10mL dichloromethane before adding dropwise), drop After the completion, continue to control the temperature at -5°C to carry out the acylation reaction for 16 hours. After the acylation reaction is completed, add 30mL of 10% dilute hydrochloric acid solution for washing. The purpose of treating with dilute hydrochloric acid is to remove unreacted Complete basic catalyst pyridine, stand still, separate layers, collect the ...
Embodiment 3
[0055] 1,4-Dibutyryl-3,6-diphenyl-1,4-dihydro-s-tetrazine (Ⅰ-3)
[0056]
[0057] The preparation method of the above-mentioned 1,4-dibutyryl-3,6-diphenyl-1,4-dihydro-s-tetrazine compound is as follows:
[0058] Add 0.8g (3.4mmol) of 3,6-diphenyl-1,2-dihydro-s-tetrazine and 0.70g (6.97mmol) of basic catalyst triethylamine into the reactor, and then add 30mL ethyl acetate Ester solvent, under the condition of stirring, lower the temperature to -10°C, then start to add 0.76g (7.14mmol) of butyryl chloride dropwise (as a preference, first dissolve 0.76g of butyryl chloride in 10mL of ethyl acetate and then add dropwise), drop After the addition is complete, continue to control the temperature at -10°C to carry out the acylation reaction for 20 hours. After the acylation reaction is completed, add 40 mL of 10% by mass aqueous dilute hydrochloric acid solution to wash and remove the unreacted basic catalyst. Ethylamine, stand still, separate layers, collect the organic phase, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com