Method for culturing mesenchymal stem cell in vitro
A technology of mesenchymal stem cells and in vitro culture, applied in the field of cell engineering, can solve the problems of insufficient cell viability for clinical and scientific research, slow cell growth, high cost, etc., and achieve the effect of high viability cell source, high cell viability, and simple configuration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of mesenchymal stem cells using the method of the present invention
[0030] Primary culture:
[0031] Using 1.073g / cm 3 Mononuclear cells were isolated from bone marrow with Percoll separation medium by gradient density centrifugation, and the mononuclear cells were inoculated into α-MEM medium containing 10% fetal bovine serum by volume at 37°C and 5% CO 2Adhesive culture was carried out under concentration conditions, and the primary mesenchymal stem cells were obtained until the adherent cells appeared and showed the shape of fibroblasts.
[0032] α-MEM culture medium: anhydrous calcium chloride 200mg / L, potassium chloride 400mg / L, anhydrous magnesium sulfate 97.67mg / L, sodium chloride 6800mg / L, sodium dihydrogen phosphate monohydrate 140mg / L, L -Alanine 25mg / L, L-arginine hydrochloride 126.98mg / L, L-asparagine 50mg / L, L-aspartic acid 30mg / L, L-cysteine hydrochloride 100mg / L, L - Cystine hydrochloride 31.28mg / L, L-glutamic acid 75mg / L, L-...
Embodiment 2
[0035] Example 2: Identification of mesenchymal stem cells of the present invention
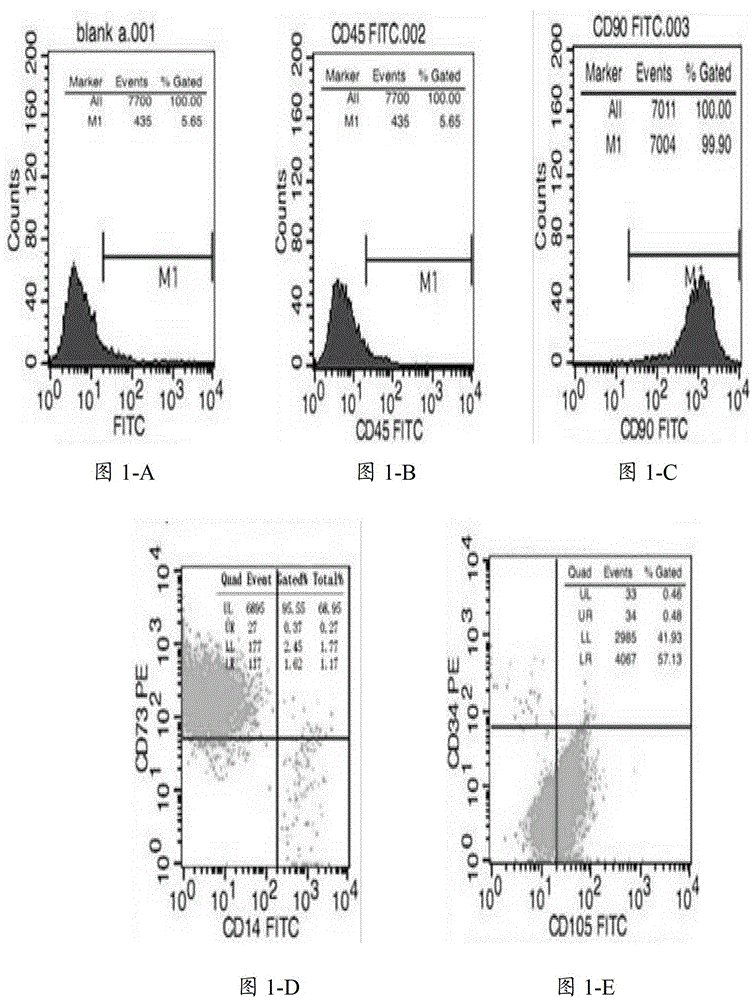
[0036] 1. Immunophenotyping
[0037] To prepare primary mesenchymal stem cells, take 1×10 4 More than one cells were added to different EP tubes, and PE or FITC-labeled anti-CD14, CD34, CD45, CD73, CD90, CD105 monoclonal antibodies were added to each tube and detected by flow cytometry, the results are shown in figure 1 . Depend on figure 1 It can be seen that the mesenchymal stem cells cultured in the present invention have no or low expression of CD14, CD34, and CD45, and high expression of CD73, CD90, and CD105, which meet the reference standards for mesenchymal stem cells proposed by the International Society for Cell Therapy.
[0038] 2. Morphological characteristics
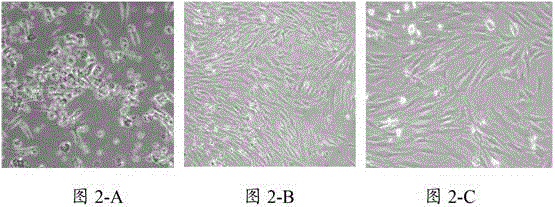
[0039] The primary mesenchymal stem cells and the third generation mesenchymal stem cells cultured in the present invention were observed under a microscope, and the results are shown in figure 2 ,Depend on figure ...
Embodiment 3
[0045] Example 3: Colony Cloning Test of Mesenchymal Stem Cells
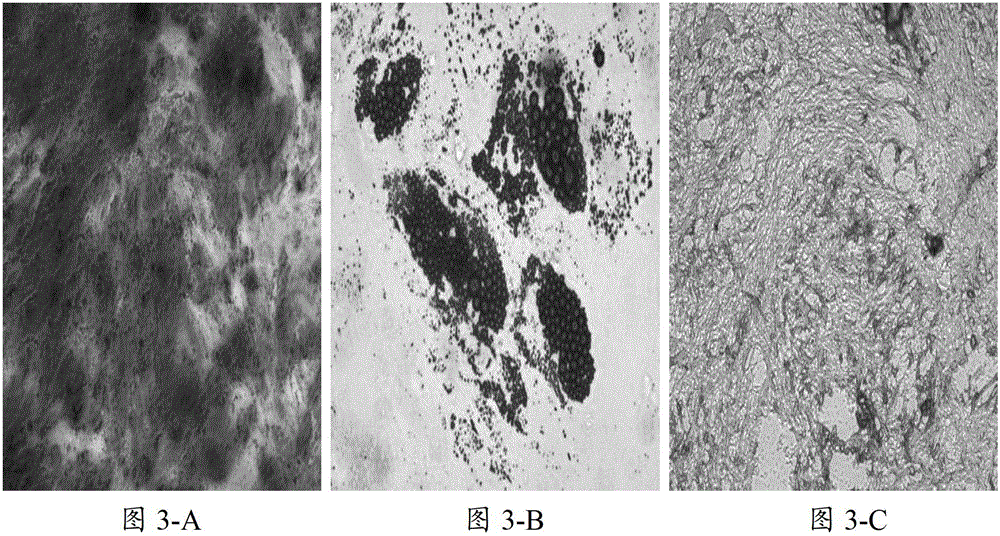
[0046] The mesenchymal stem cells cultured by the method described in the present invention and the Dexter-LTC culture system were subjected to colony clone hematoxylin staining (the culture environment was consistent), and statistical and significant difference analysis was performed (≥50 cells were counted as a colony), The results are shown in Table 1 and Figure 4 .
[0047] Table 1 Count of mesenchymal stem cell colony clone test
[0048] group
Number of colonies
The average number of cells in a colony
Dexter-LTC culture system
8.0±1.0
59.2±8.2
The method of the invention
11.3±1.53*
66.5±14.4*
[0049] Note: * means there is a significant difference compared with the Dexter-LTC system (P<0.05)
[0050] It can be seen from Table 1 that the number of mesenchymal stem cell colonies cultured according to the method of the present invention and the average...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com