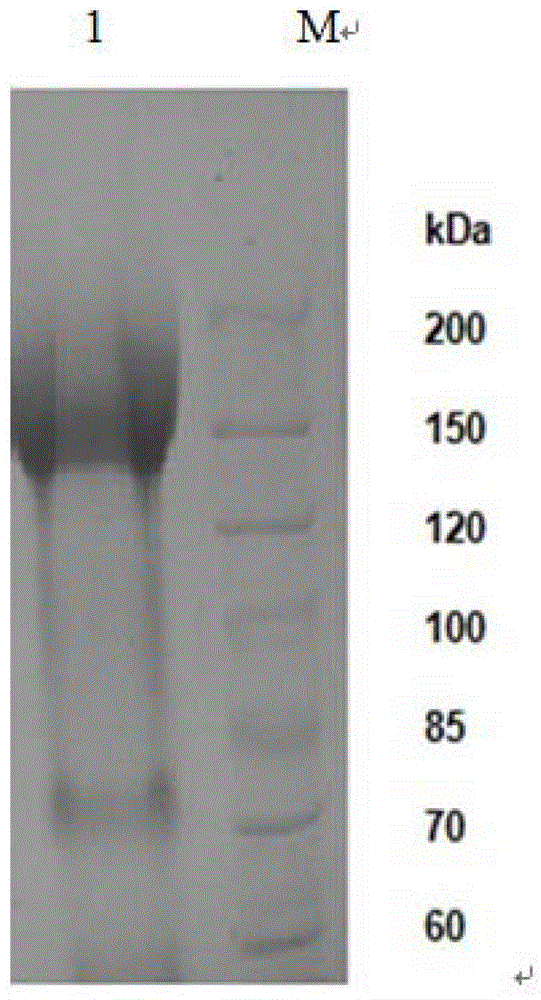
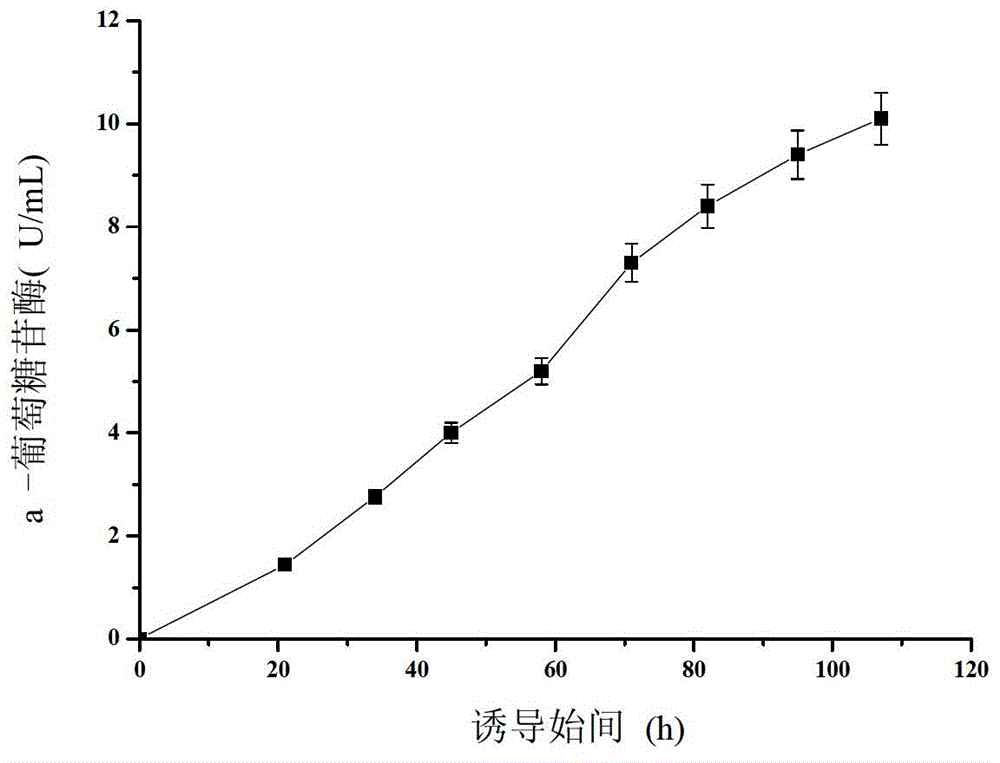
Aspergillus niger alpha-glucosidase gene and high-efficiency expression method thereof
A technology of glucosidase and Aspergillus niger, which is applied in the field of enzyme engineering and can solve problems such as insufficient expression level to meet production needs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: This example illustrates the codon optimization process for alpha-glucosidase.
[0017] 1. Optimization and synthesis of AGL gene:
[0018] Based on the α-glucosidase sequence of A. niger CBS513.88 in the NCBI database (NCBI accession number: 4991096), its codon usage frequency was analyzed by Graphical Codon Usage Analyzer (http: / / gcua.schoedl.de / ) , using DNAWorks software to optimize the Aspergillus niger α-glucosidase gene sequence. DNAMAN was used to analyze the restriction endonuclease sites of the target gene. The optimized gene was synthesized by Shanghai Sangon Biotechnology Co., Ltd. Our strategy is to replace rare codons with favored codons. A total of 728 bases were changed, involving 222 codons. The GC content decreased from 50.0% to 44.2%. The homology between the synthetic sequence and the original sequence was 74.0%.
[0019] Optimized α-glucosidase gene aglu OP The nucleotide sequence is shown as SEQ ID No1.
[0020] 2. Cloning and scree...
Embodiment 2
[0025] Example 2: This example illustrates recombinant P. pastoris KM71 / pPIC9K-aglu OP Strain 3L tank fermentation culture
[0026] Draw 200μL of the glycerol tube bacteria solution and inoculate it into a 500mL Erlenmeyer flask containing 50mL of seed medium, cultivate at 30°C and 200r / min for 16-20h, and insert the above culture solution with 10% inoculum volume into 0.8L A 3L fermenter (BIOFLO, America), controlled pH5 with 25% ammonia water, cultured at 30°C, and maintained dissolved oxygen at about 20% by coupling with stirring speed and adjusting ventilation. When dissolved oxygen rose rapidly, it indicated that the culture medium The glycerol in the medium has been exhausted, and the glycerol feeding solution is started to be fed exponentially. When the bacterial cell concentration reaches 115.2g / L, the feeding is stopped, and the dissolved oxygen rebounds again. Set the induction temperature at 26°C and add 2% methanol. After the recombinant P. pastoris adapts to meth...
Embodiment 3
[0027] Example 3: This example illustrates the co-expression of the P. pastoris disulfide isomerase pdi gene
[0028] The recombinant expression vector pPICZA-pdi constructed earlier in our laboratory was linearized by Sac I digestion and then electroporated into P. pastoris KM71 / pPIC9K-aglu OP In , the transformants were able to grow on basal medium because the cells already contained the G418 resistance gene and the pPIC9K vector integrated into the chromosome. In order to improve the screening efficiency, spread the above-mentioned competent cells on the MD plate containing 0.5 mg / mL G418 resistance concentration, and then pick a single colony and inoculate it on the YPD plate containing gradient Zeocin resistance. The Zeocin resistance gradient was 0.25mg / mL, 0.5mg / mL, 1mg / mL, 2mg / mL. Pick a single colony on a 2 mg / mL Zeocin-resistant plate with a toothpick and inoculate it in 10 mL of YPD medium, culture at 30°C overnight, and store in a glycerol tube. Then take 1mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com