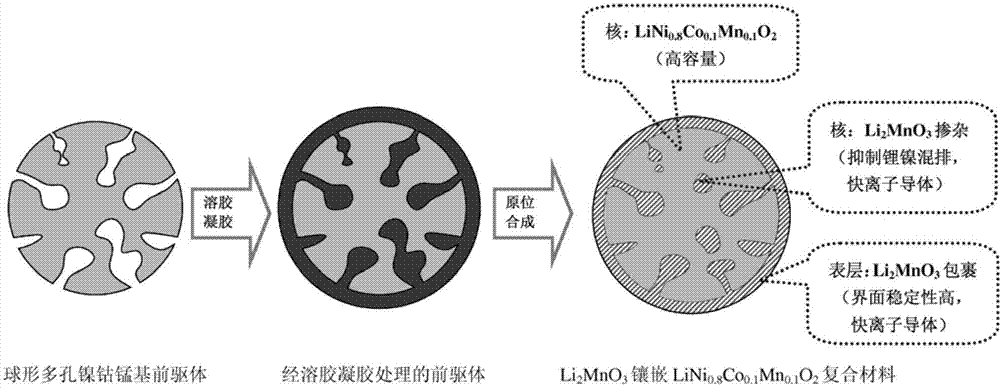
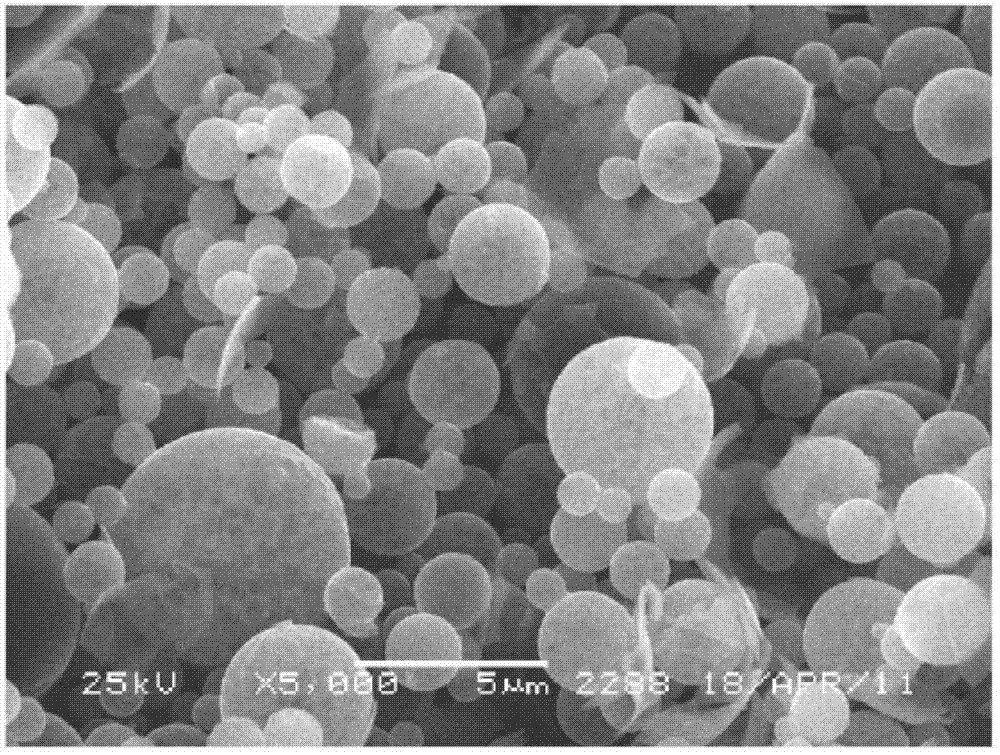
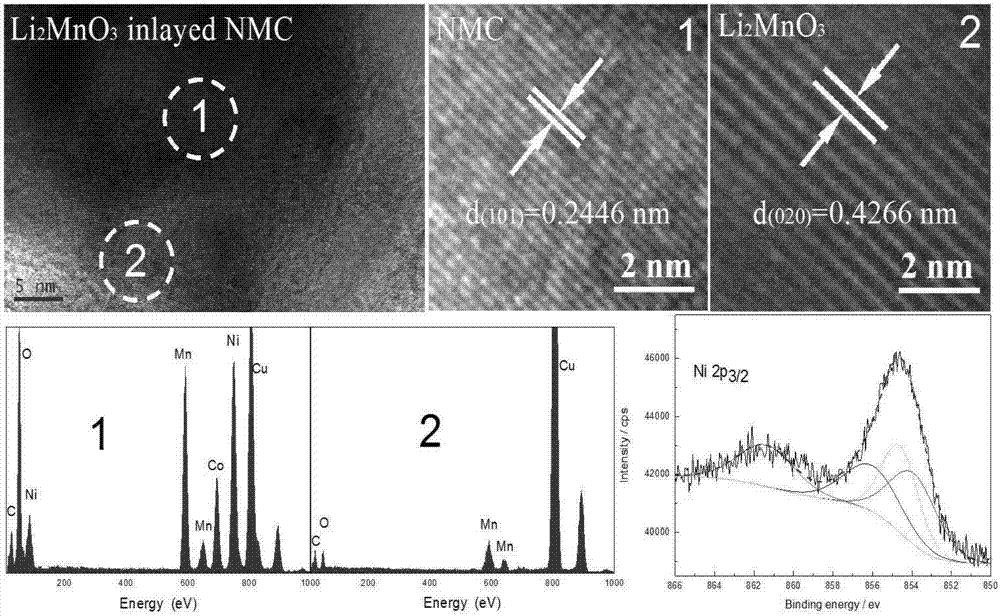
In-situ synthesis method of fast ion conductor inlaid lithium ion battery cathode material
A lithium-ion battery, in-situ synthesis technology, applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems of lower interface conductivity, low coulombic efficiency of first charge and discharge, increase of interface impedance, etc., and achieve strong chemical stability and thermal stability, improving cycle performance and safety, and widening the stable voltage range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]1) Preparation of spherical porous nickel-cobalt M-based precursor: the nickel, cobalt, and manganese acetates are mixed into a 0.4mol / L nickel-cobalt-manganese mixed solution according to the nickel, cobalt, and manganese molar ratio of 8:1:1 ; Citric acid is added in the mixed solution by 1 times of the total molar amount of nickel, cobalt and manganese added. Pass the above mixed solution into a spray dryer with an inlet air temperature of 200°C and an outlet air temperature of 100°C. After atomization and drying, the precursor powder (with a particle size of 1-20 μm) is collected. The precursor was placed in a muffle furnace and kept at 200°C for 3 hours in an air atmosphere, and a spherical porous nickel-cobalt-manganese-based precursor was obtained after cooling.
[0030] 2), Li 2 MnO 3 Mosaic LiNi a co b m 1-a-b o 2 Preparation of composite positive electrode material: the acetate of citric acid, lithium and manganese is formulated into a mixed solution, whe...
Embodiment 2
[0033] 1) Preparation of spherical porous nickel-cobalt M-based precursor: the nickel, cobalt, and manganese acetates are mixed into a 0.1mol / L nickel-cobalt-aluminum mixed solution according to the nickel, cobalt, and aluminum molar ratio of 8:1.5:0.5 ; Citric acid is added in the mixed solution by 3 times of the total molar amount of nickel, cobalt and aluminum added. Pass the above mixed solution into a spray dryer with an inlet air temperature of 150°C and an outlet air temperature of 80°C. After atomization and drying, the precursor powder (with a particle size of 1-20 μm) is collected. The precursor was placed in a muffle furnace and kept at 150° C. for 6 hours in an air atmosphere, and a spherical porous nickel-cobalt-manganese-based precursor was obtained after cooling.
[0034] 2), Li 2 MnO 3 Mosaic LiNi a co b m 1-a-b o 2 Preparation of composite positive electrode material: the acetate of citric acid, lithium and manganese is formulated into a mixed solution, ...
Embodiment 3
[0037] 1), the preparation of spherical porous nickel-cobalt M-based precursor: the acetate of nickel, cobalt, and manganese is mixed into a 2mol / L nickel-cobalt-manganese mixed solution according to the molar ratio of nickel, cobalt, and manganese of 8:1:1; Citric acid is added in the mixed solution by 1 times of the total molar amount of nickel, cobalt and manganese added. Pass the above mixed solution into a spray dryer with an inlet air temperature of 300°C and an outlet air temperature of 150°C. After atomization and drying, the precursor powder (with a particle size of 1-20 μm) is collected. The precursor was placed in a muffle furnace and kept at 400° C. for 0.5 h in an air atmosphere, and a spherical porous nickel-cobalt-manganese-based precursor was obtained after cooling.
[0038] 2), Li 2 MnO 3 Mosaic LiNi a co b m 1-a-b o 2 Preparation of composite positive electrode material: the acetate of citric acid, lithium and manganese is formulated into a mixed soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com