Ferrite/graphene composite adsorbent and preparation and using methods thereof
A graphene composite, ferrite technology, applied in chemical instruments and methods, adsorption water/sewage treatment, other chemical processes, etc., can solve the decline of heavy metal ion adsorption capacity, the limitation of graphene preparation and application, and the easy occurrence of agglomeration, etc. The problem is to reduce the surface energy, the method is simple, and the energy consumption is reduced.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
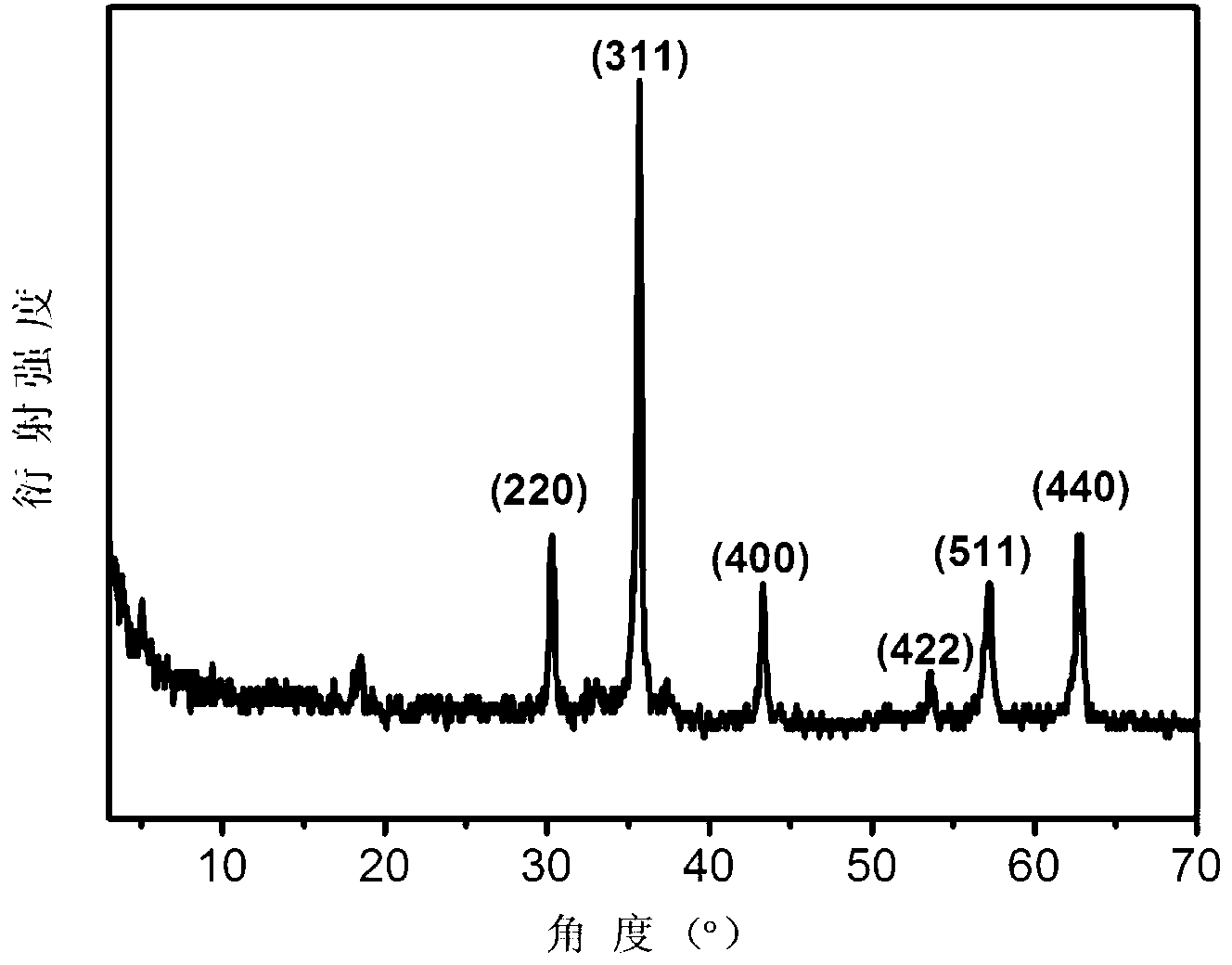
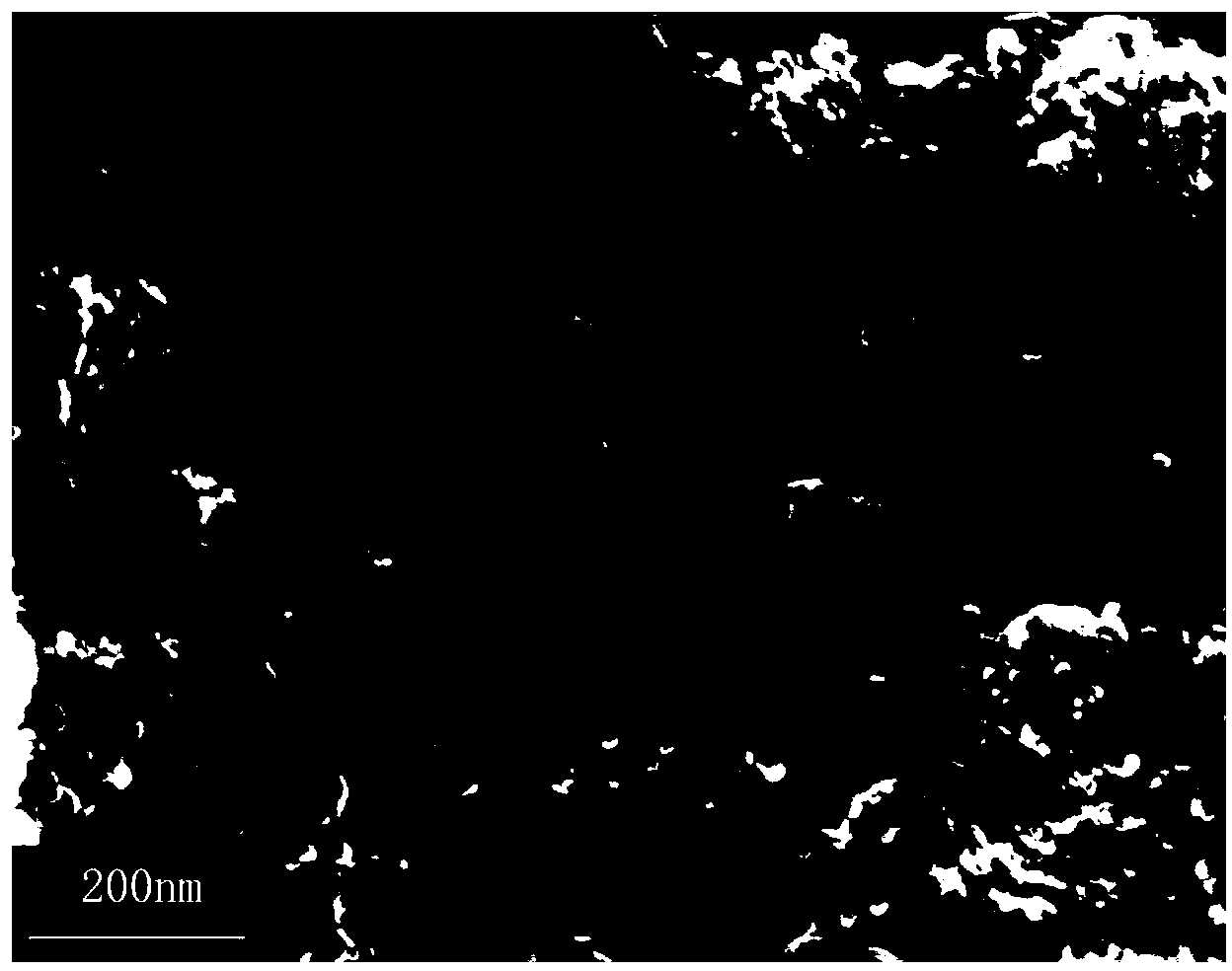
[0022] Accurate weighing of MnCl according to stoichiometric ratio 2 4H 2 O and Fe(NO 3 ) 3 9H 2 O, dissolved in 2.0 g / L graphite oxide solution, Mn in the solution2+ The concentration of metal ions is 0.1 M, Fe 3+ The concentration of metal ions is 0.2 M; another hexamethylenetetramine solution is prepared, [C 6 h 12 N 4 ]=1.0M. The graphite oxide solution containing the metal salt and the hexamethylenetetramine solution were transferred into a polytetrafluoroethylene liner, and the hydrothermal crystallization reaction was carried out at 120 °C for 10 hours. After naturally cooling to room temperature, centrifuge and wash to obtain MnFe 2 o 4 / graphene adsorbent. Among them, the mass percentage of ferrite is 34%, the size distribution of ferrite nanoparticles is 9nm, and the specific surface area of the composite adsorbent is 269 m 2 / g;
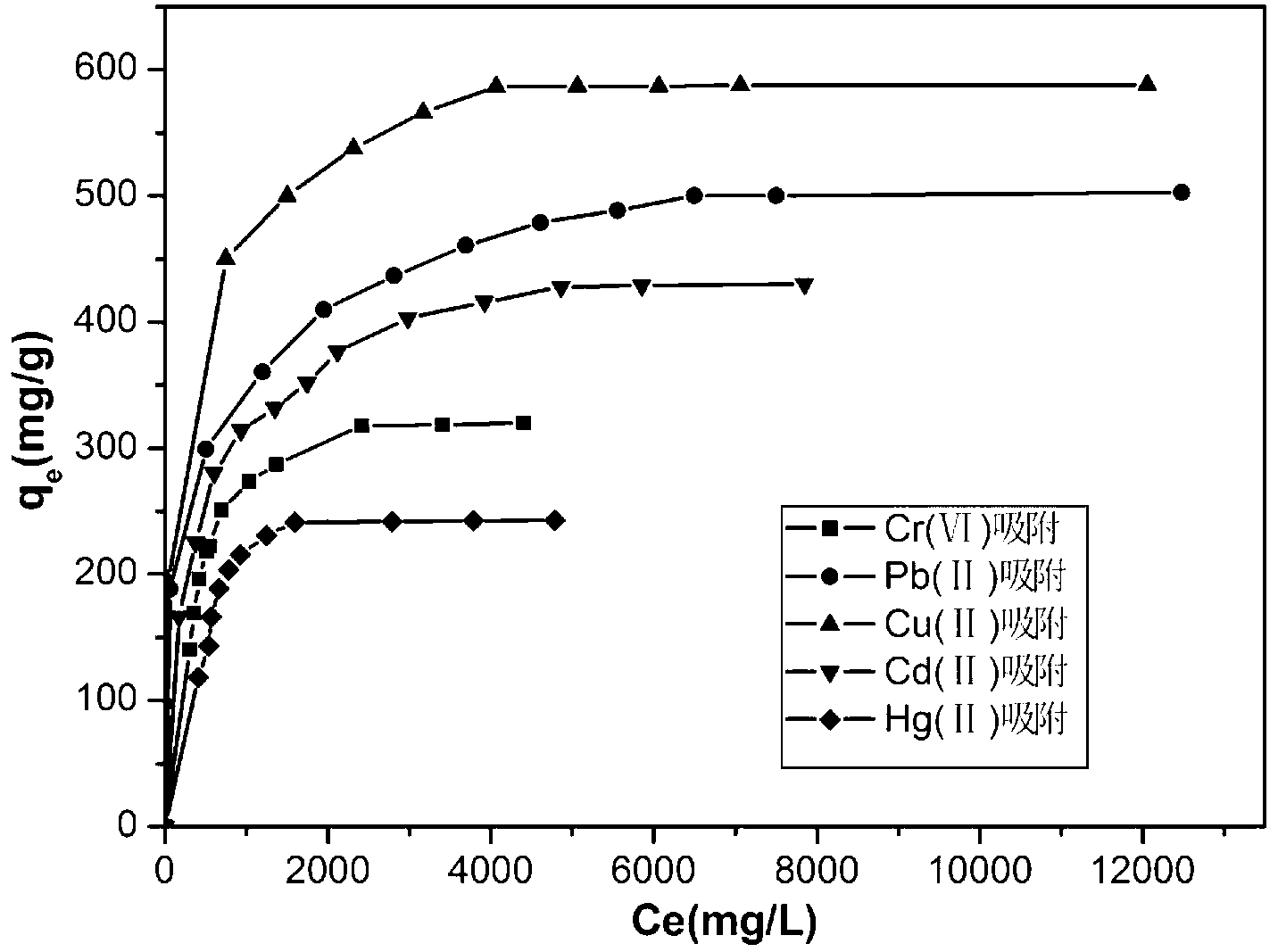
[0023] The above 0.05g ferrite / graphene composite adsorbent was dispersed in the Cr-containing 6+ , Pb 2+ , Cu 2+ , Cd ...
Embodiment 2
[0025] Accurately weigh Ni(NO 3 ) 2 ·6H 2 O and Fe(NO 3 ) 3 9H 2 O, dissolved in 1.5g / L graphite oxide solution, Ni in the solution 2+ The concentration of metal ions is 0.2 M, Fe 3+ The concentration of metal ions is 0.4 M; another hexamethylenetetramine solution is prepared, [C 6 h 12 N 4 ]=2.0M. The graphite oxide solution containing the metal salt and the hexamethylenetetramine solution were transferred into a polytetrafluoroethylene liner, and the hydrothermal crystallization reaction was carried out at 150 °C for 12 hours. After naturally cooling to room temperature, centrifuge and wash to obtain NiFe 2 o 4 / graphene adsorbent. Among them, the mass percentage of ferrite is 45%, the size distribution of ferrite nanoparticles is 18nm, and the specific surface area of the composite adsorbent is 251 m 2 / g;
[0026] The above 0.05g ferrite / graphene composite adsorbent was dispersed in the Cr-containing 6+ , Pb 2+ , Cu 2+ , Cd 2+ , Hg 2+ The solution was...
Embodiment 3
[0028] Accurately weigh Co(NO 3 ) 2 ·6H 2 O and Fe(NO 3 ) 3 9H 2 O, dissolved in 1.0 g / L graphite oxide solution, Co in the solution 2+ The concentration of metal ions is 0.3M, Fe 3+ The concentration of metal ions is 0.6 M; another hexamethylenetetramine solution is prepared, [C 6 h 12 N 4 ]=3.0M. The graphite oxide solution containing the metal salt and the hexamethylenetetramine solution were transferred into a polytetrafluoroethylene liner, and the hydrothermal crystallization reaction was carried out at 180 °C for 16 hours. After naturally cooling to room temperature, centrifuge and wash to obtain CoFe 2 o 4 / graphene adsorbent. Among them, the mass percentage of ferrite is 56%, the size distribution of ferrite nanoparticles is 20nm, and the specific surface area of the adsorbent is 203 m 2 / g;
[0029] The above 0.05g ferrite / graphene composite adsorbent was dispersed in the Cr-containing 6+ , Pb 2+ , Cu 2+ , Cd 2+ , Hg 2+ The solution was adsorbed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size distribution | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com