Multi-cyandiamine monomer and preparation and application thereof
The technology of polycyanodiamine monomer and polycyanopolyimide is applied in the fields of polycyanodiamine-containing monomer and its preparation and application, and can solve the problem of separation of material and matrix, poor adsorption performance, sulfonation high degree issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
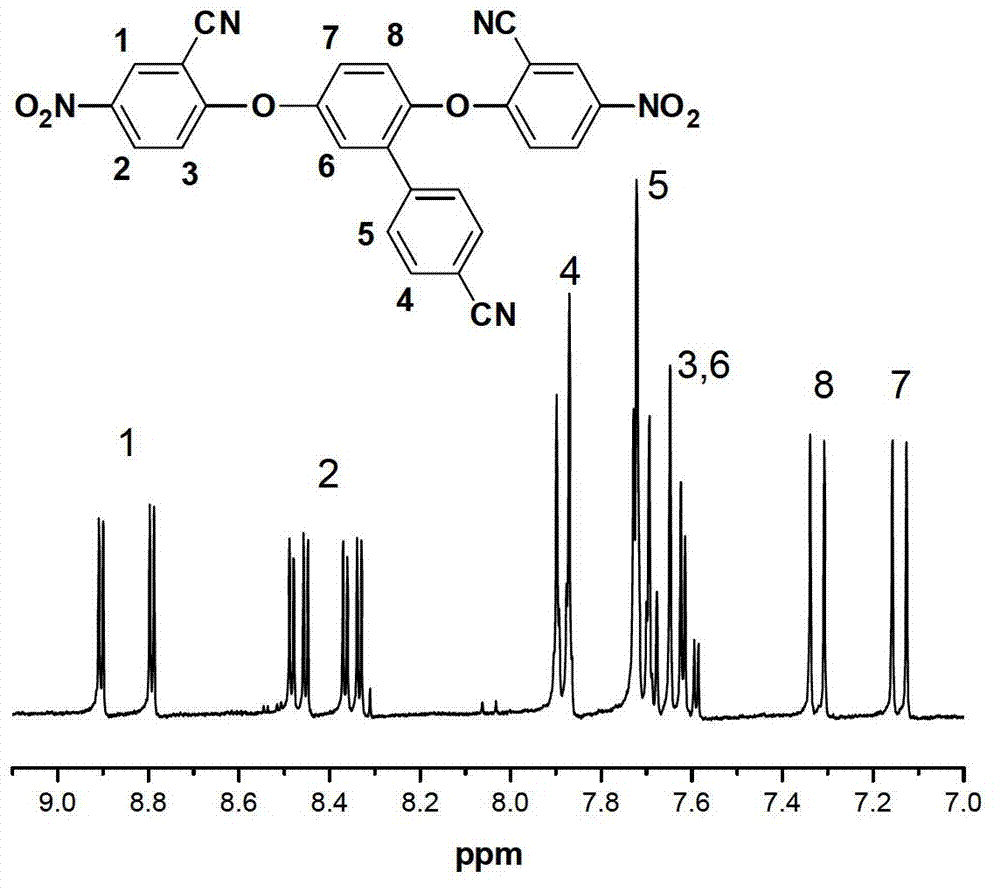
[0066] (1) Add 3.168g (0.015mol) of 4-cyanophenylhydroquinone, 2-chloro-5-nitrobenzene to a three-necked flask equipped with an electric stirring device, nitrogen protection device and water dispenser Nitrile 5.751g (0.0315mol) and anhydrous potassium carbonate 2.346g (0.017mol), 45mL (43g) DMF and 15mL toluene, stirred and reacted at 132-135°C for 2-3 hours, after the water was completely removed, the water and toluene were released ; Heating to 148-152°C and continuing to stir for 3-4 hours; Pour the reactant into a mixture of ice water and ethanol to obtain a large amount of yellow precipitate; After filtering, wash with pure water several times, and dry the product in vacuum at 80°C to obtain polycyanide Dinitromonomer 2,5-bis(4-nitro-2-cyanophenoxy)-1-benzonitrile, i.e. tricyanodinitromonomer, the yield is 90%~95% ;
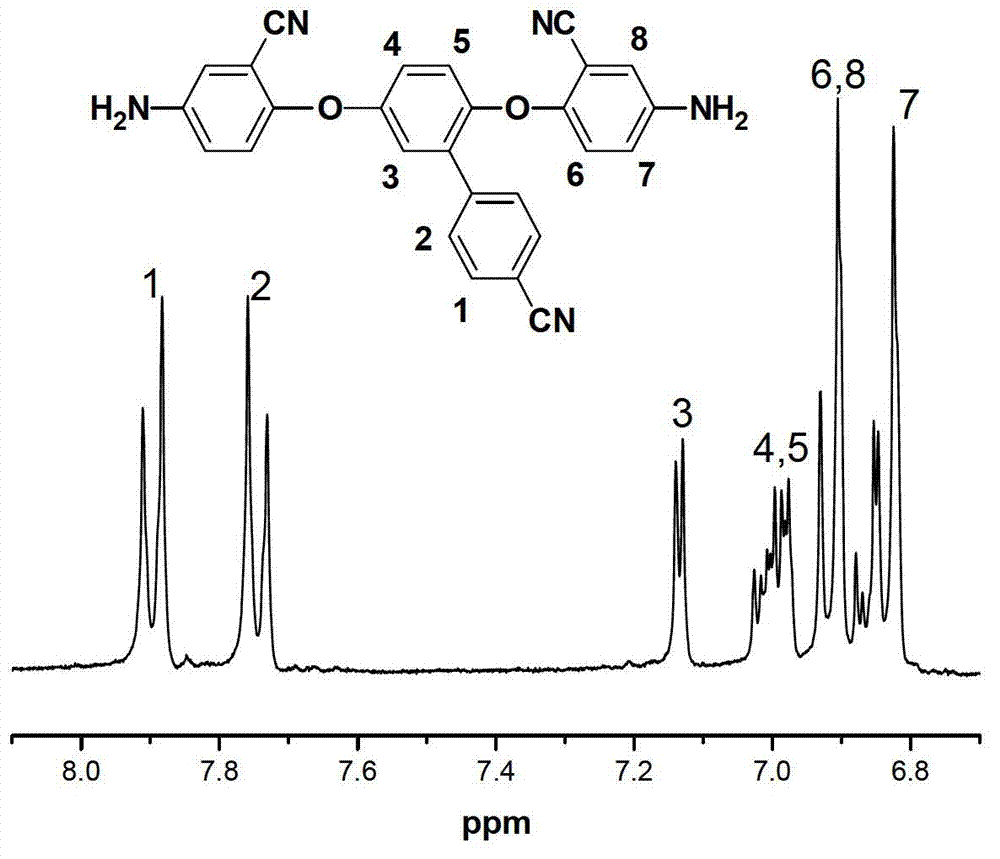
[0067] (2) Add the 2,5-bis(4-nitro-2-cyanophenoxy)-1-benzene Formaldehyde 5.03g (0.01mol), 0.2g of 10% palladium carbon catalyst and 100mL of absolute eth...
Embodiment 2
[0071] Change the consumption of reactant, repeat embodiment 1. The consumption of all reactants is 2 times of implementing 1.
[0072] The characterization results of tricyanodinitro monomer and tricyanodiamine monomer are similar to those in Example 1.
Embodiment 3
[0074] Repeat Example 2 with 7.087g (0.03mol) 3,4-2 cyanophenyl hydroquinone instead of 4-cyanophenyl hydroquinone to synthesize tetracyanodiamine monomer 2,5-di( 4-Amino-2-cyanophenoxy)-1-o-dibenzonitrile, in step (2), the intermediate polycyanodinitromonomer 2,5-bis(4-nitro-2 -cyanophenoxy)-1-o-dibenzonitrile (abbreviated as tetracyanodinitromonomer) 10.56g; the tetracyanodiamine monomer 2,5-bis(4-amino-2 -cyanophenoxy)-1-o-dibenzonitrile (i.e. tetracyanodiamine monomer) has a melting point of 236°C and a yield of 82%; its structural formula is as follows:
[0075]
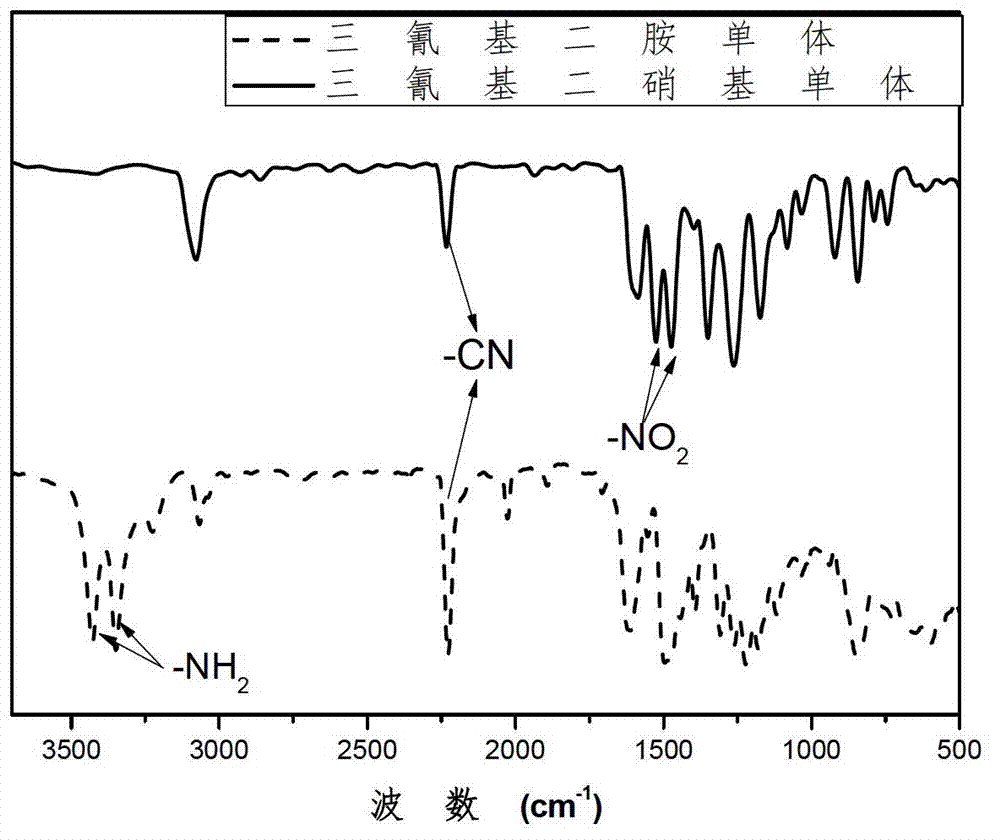
[0076] From Figure 4 It can be seen that the infrared characteristic peaks of tetracyanodinitro monomer and tetracyanodiamine monomer are similar to the tricyano monomer in Example 1, which proves that the present invention has prepared tetracyanodiamine monomer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com