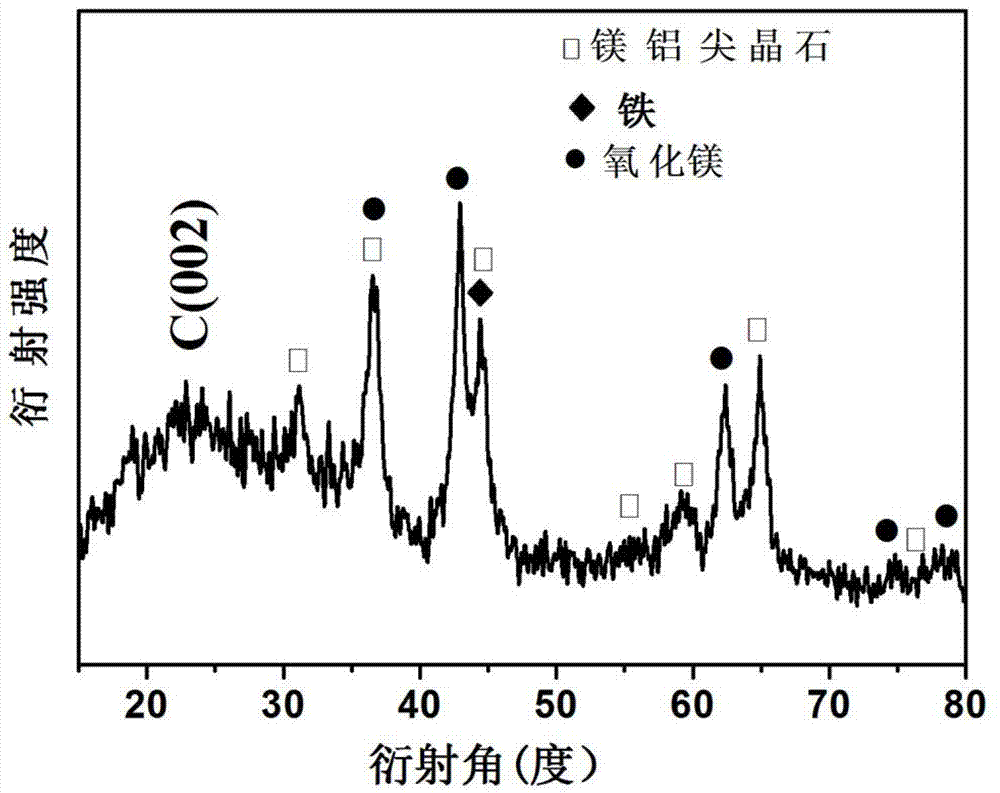
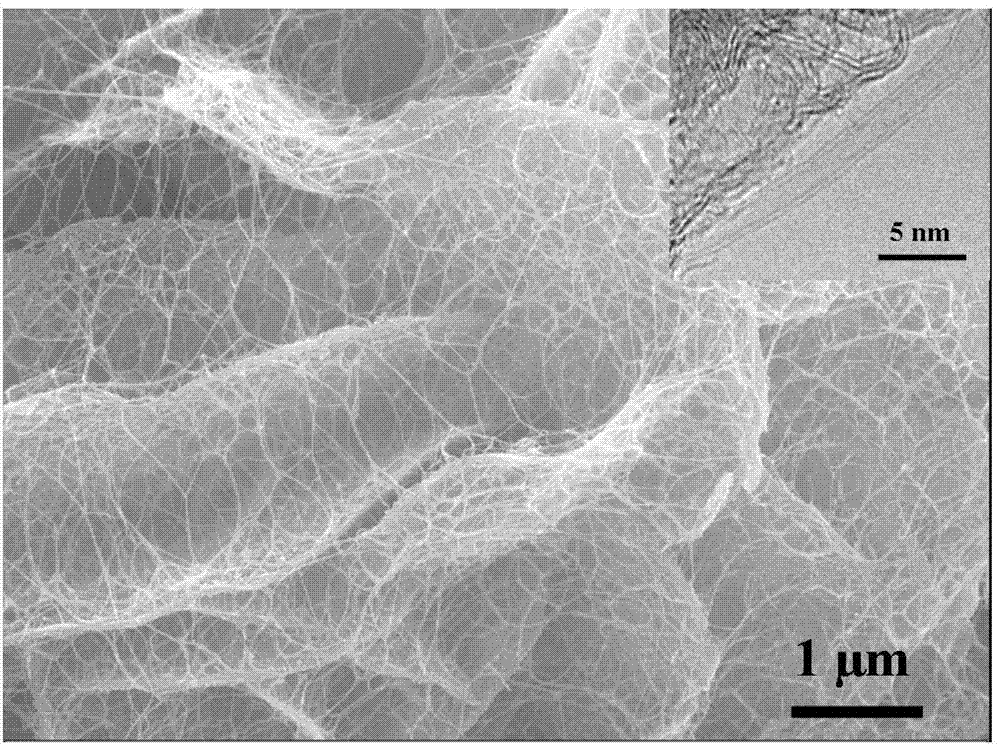
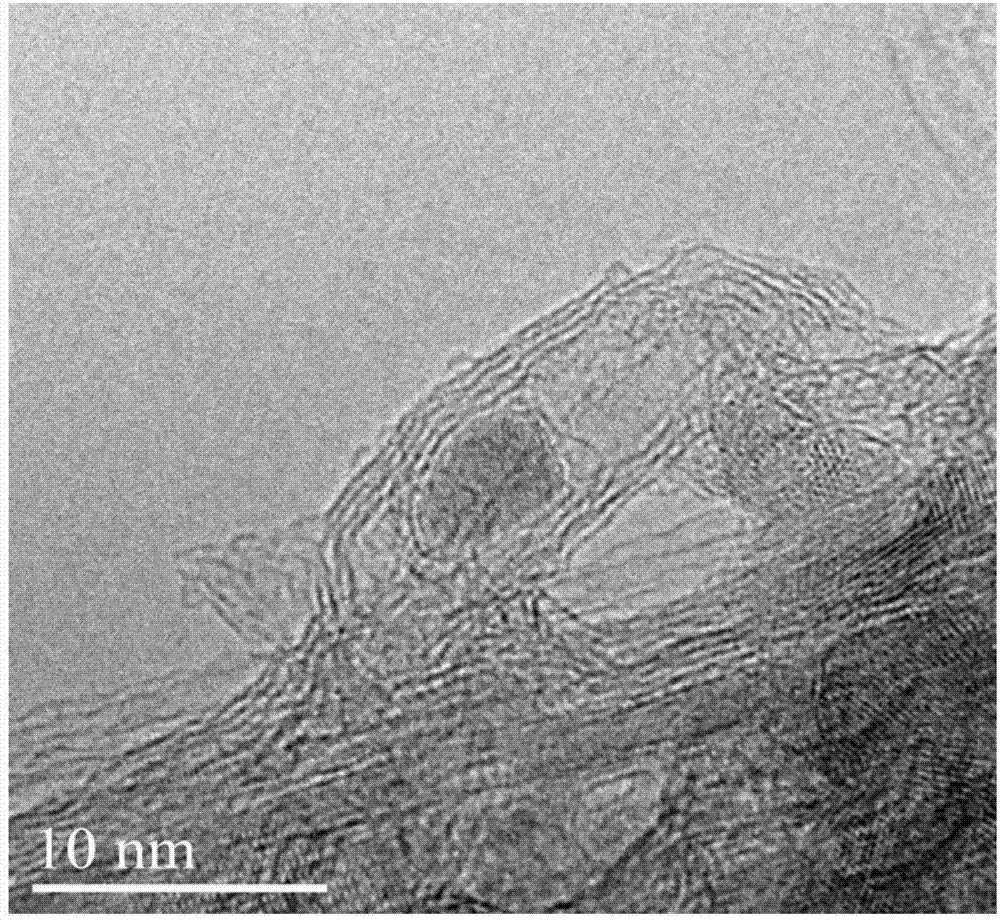
Preparation method of high-dispersion supported nano metal Fe-based catalyst
A nano-metal, high-dispersion technology, applied in the direction of metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of reducing catalytic performance and easy agglomeration of metal particles. Achieve the effects of inhibiting aggregation, improving chemical stability, and good catalytic oxidation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 3.8461g of Mg(NO 3 ) 2 ·6H 2 O and 5.6270g of Al(NO 3 ) 3 ·9H 2 O is prepared into 50ml mixed solution; weigh 0.4938g of K 3 [Fe(CN) 6 ] Add to the above mixed solution. Weigh 3.7537g urea and dissolve it in 50ml methanol aqueous solution (V CH3OH / V H2O = 1:3). Transfer the prepared mixed salt solution and alcohol aqueous solution to a polytetrafluoroethylene crystallization reactor, seal and heat to 100°C, react for 6 hours and then cool to room temperature and let stand for 12 hours, take out the mixture and centrifuge, and centrifuge with deionized water Wash 4 times, dry for 24h in air atmosphere, and obtain intercalated LDHs. Spread 0.5g of intercalated LDHs evenly in a small porcelain boat, and place the small porcelain boat in the middle of the quartz tube of the tubular atmosphere furnace, and introduce N with a flow rate of 300ml / min 2 , Starting from 30°C, increasing to 900°C at a heating rate of 5°C / min, and then introducing N with a flow rate of 600ml / min ...
Embodiment 2
[0027] Mix 1.8485g of MgSO 4 ·7H 2 O and 3.3308g Al 2 (SO 4 ) 3 ·18H 2 O is made into 50ml mixed solution; Weigh 0.2469g of K 3 [Fe(CN) 6 ] Add to the above mixed solution. Weigh 2.2522g urea and dissolve it in 50ml ethanol aqueous solution (V C2H5OH / V H2O = 1:4). Transfer the prepared mixed salt solution and alcohol aqueous solution to a polytetrafluoroethylene crystallization reactor, seal and heat to 120°C, react for 4 hours and then cool to room temperature and let stand for 24 hours, take out the mixture and centrifuge, and centrifuge with deionized water It was washed 4 times and dried for 36 hours under air atmosphere to obtain intercalated LDHs. Spread 0.5g of intercalated LDHs evenly in a small porcelain boat, and place the small porcelain boat in the middle of the quartz tube of the tubular atmosphere furnace, and pass N with a flow rate of 400ml / min. 2 , Starting from 30°C, increasing to 800°C at a heating rate of 3°C / min, and then introducing N with a flow rate...
Embodiment 3
[0030] Add 4.066g of MgCl 2 ·6H 2 O and 4.2632g of AlCl 3 ·6H 2 O is made into 50ml mixed solution; Weigh 0.8231g of K 3 [Fe(CN) 6 ] Add to the above mixed solution. Weigh 4.5045g urea and dissolve it in 50ml methanol aqueous solution (V CH3OH / V H2O = 1:5). Transfer the prepared mixed salt solution and alcohol aqueous solution to a polytetrafluoroethylene crystallization reactor, seal and heat to 140℃, react for 5 hours and then cool to room temperature and let stand for 18 hours, take out the mixture and centrifuge, and centrifuge with deionized water It was washed 4 times and dried in air for 32 hours to obtain intercalated LDHs. Spread 0.5g intercalated LDHs evenly in a small porcelain boat, and place the small porcelain boat in the middle of the quartz tube of the tubular atmosphere furnace, and pass N with a flow rate of 500ml / min. 2 , Starting from 30°C, increasing to 1000°C at a heating rate of 2°C / min, and then introducing N with a flow rate of 400ml / min 2 And H 2 M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com