Human interleukin29 mature peptide mutant (hIL-29Z) with arginine and lysine produced respectively by site-directed mutagenesis of 33th lysine and 35th arginine and preparation method thereof
An interleukin, site-directed mutagenesis technology, applied in the fields of botanical equipment and methods, microorganism-based methods, biochemical equipment and methods, etc., can solve problems such as non-specificity of cell responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1. Cloning of human interleukin 29 variant encoding gene
[0022] 1. Design and Synthesis of PCR Primers
[0023] According to pPIC9K in GenBank M Carrier Factor signal peptide sequence and human IL-29 gene sequence, use Oligo7 software to design a pair of specific primers as follows:
[0024] Upstream primer: 5'- CTCGAGAAAAAGA GGCCCTGTCCCCCACTTCC-3'
[0025] Downstream primer: 5'- GCGGCCGC TCAGGTGGACTCAGGGTGG-3'
[0026] Mutation primer: 5'-TCCAATGCGTCC T TGGCC C TCTTGAAGCTCGCTA-3'
[0027] Added restriction endonuclease Xho I recognition site (CTCGAG) and protease Kex2 recognition site (GAGAAAAGA, encoding product is Glu-Lys-Arg) in the upstream primer, added Not I site (GCGGCCGC ), in order to obtain the mutation product, the 13th base in the mutation primer is designed as T, and the triplet code composed of this base is mutated from AGG to AAG, and the 35th amino acid encoded by the triplet code is composed of arginine (Arg ) is mutated to lysin...
Embodiment 2
[0030] Example 2: Construction of recombinant eukaryotic expression vectors expressing human IL-29 variants
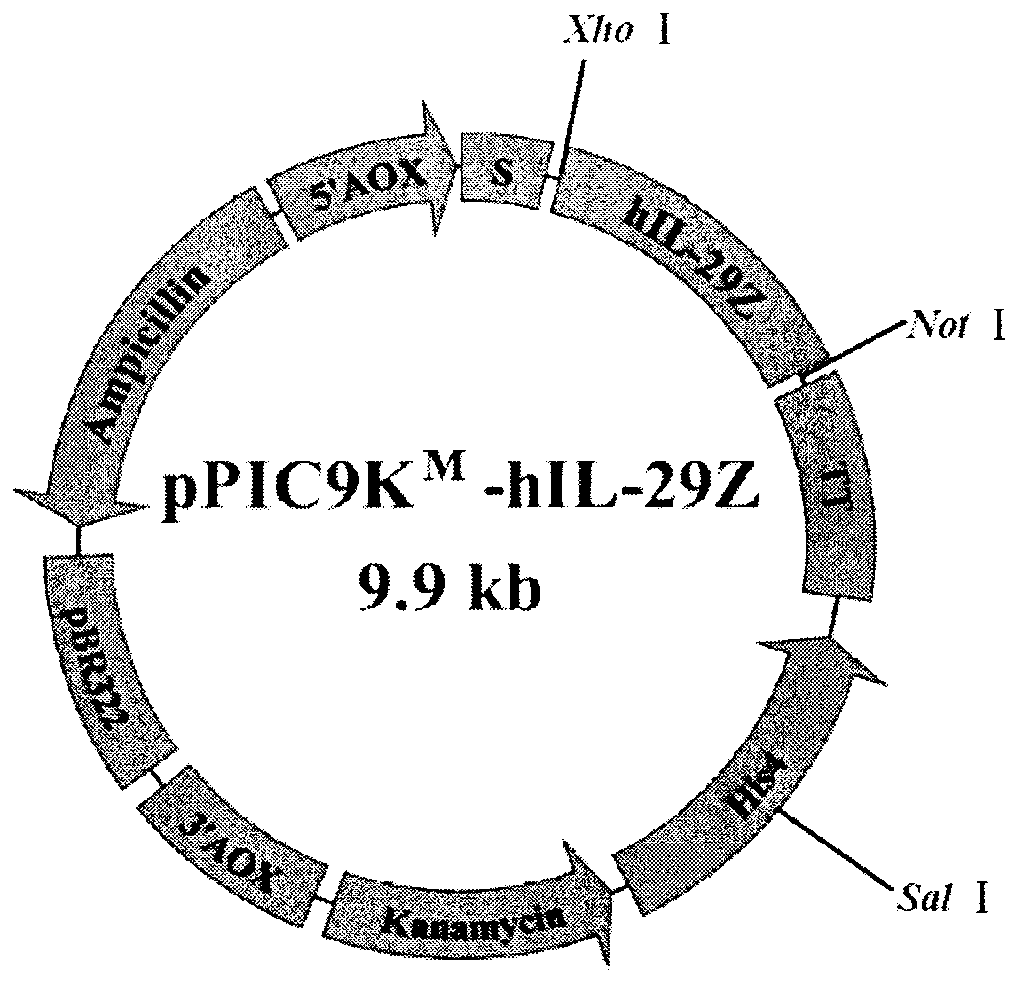
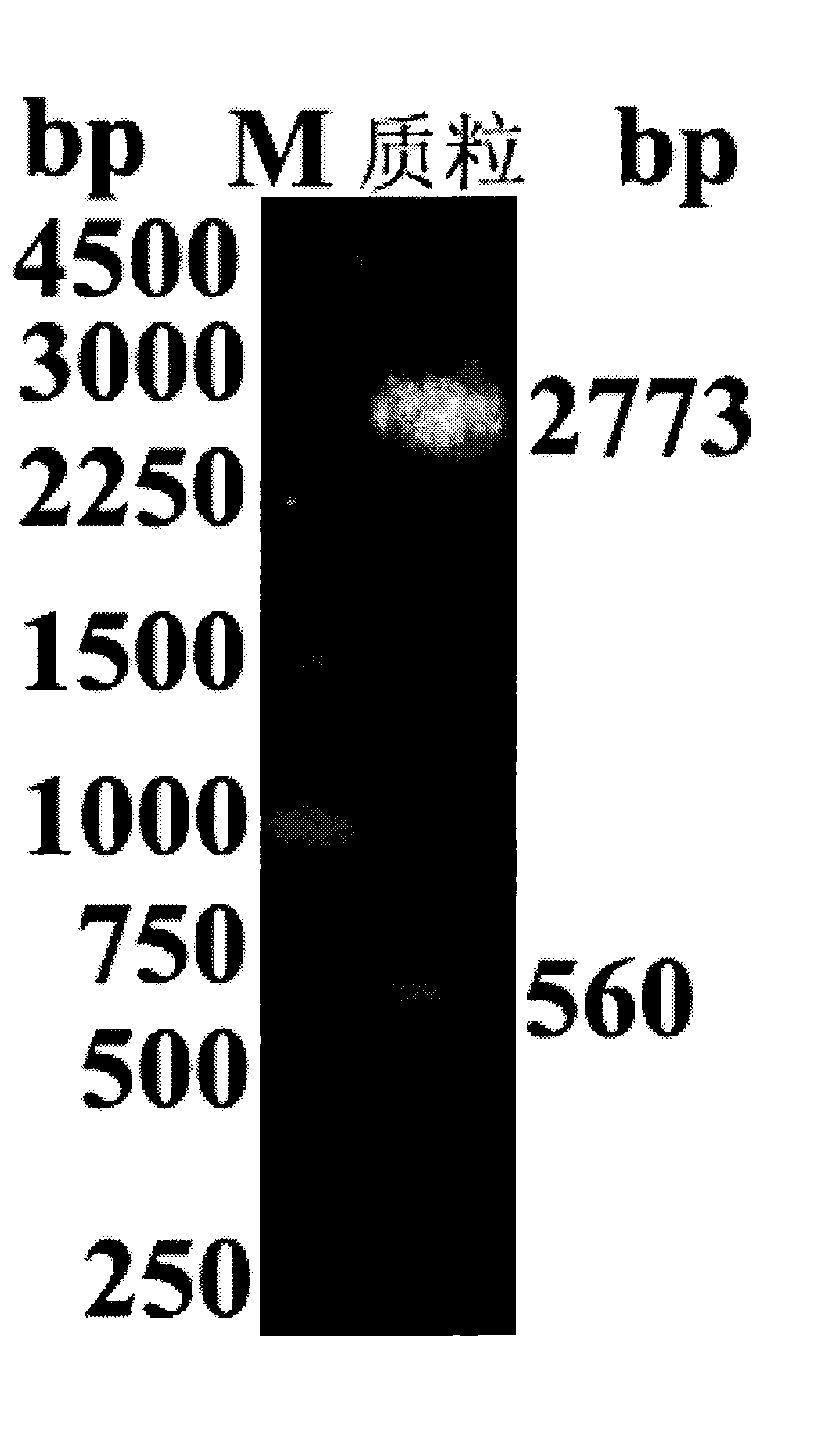
[0031] Extraction of pUCm-29Z and vector pPIC9K M (from Invitrogen Company, modified by our laboratory and applied for a patent, application number: 201110410391.0), each take 20 μL of the plasmid and perform double digestion with restriction endonuclease Xho I and NotI respectively, and recover about 560 bp of the target Gene fragment and vector pPIC9K M For large fragments, use T 4 DNA ligase (BBI Company) was connected in a water bath at 16°C for 20 hours, and the ligation product was transformed into competent Escherichia coli JM109, then inoculated on LB culture plates containing kanamycin, cultured overnight at 37°C, and positive colonies were picked to inoculate the liquid Amplify in LB medium, extract the recombinant eukaryotic expression plasmid and name it pPIC9K M -hIL-29Z; identification of recombinant eukaryotic expression plasmid pPIC9K by Xho I and No...
Embodiment 3
[0032] Example 3: Transformation of Yeast GS115 with Recombinant Eukaryotic Expression Plasmid and Screening of High Copy Recombinant Engineering Bacteria
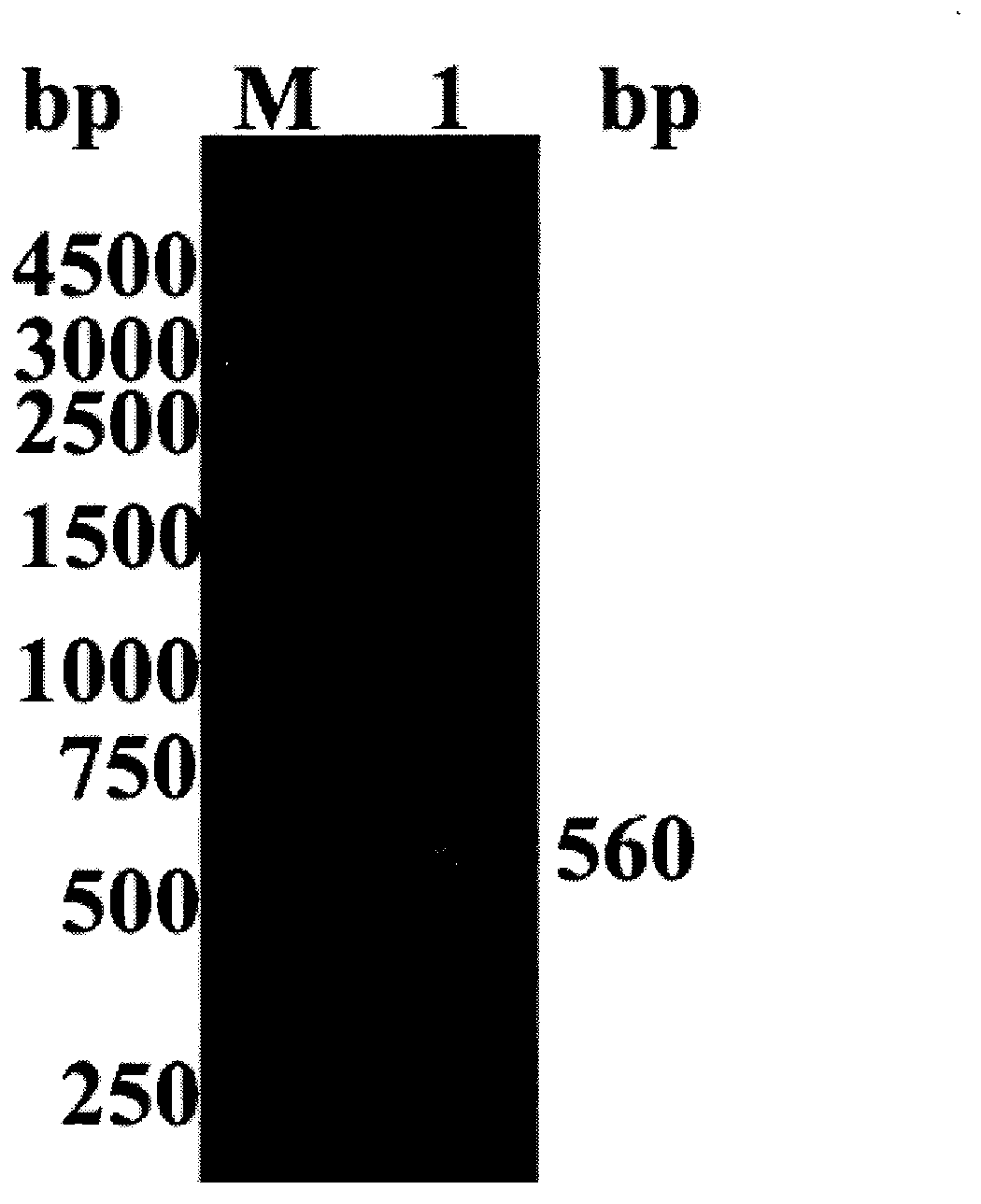
[0033] Extract pPIC9K identified by sequencing M - hIL-29Z plasmid, take 20 μL and digest it with restriction endonuclease Sal I to linearize it, separate and purify it by 0.7% agarose gel electrophoresis, and recover the linearized pPIC9K M - hIL-29Z; mixed linearized pPIC9K M - hIL-29Z and yeast GS115 competent cells were electrotransformed according to the method of the Pichia pastoris expression manual (Invitrogen).
[0034] Spread the transformation product on the MD culture plate without His, culture at 30°C for 2-3 days, select the dominant colony that grows vigorously on the MD plate and inoculate it on the YPD plate containing 0.5mg / mL G418, and culture at 30°C for 2-3 days , select the vigorously growing dominant colony and inoculate it on a YPD plate containing 1mg / mL G418, culture it at 30°C for 2 to 3 days, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com