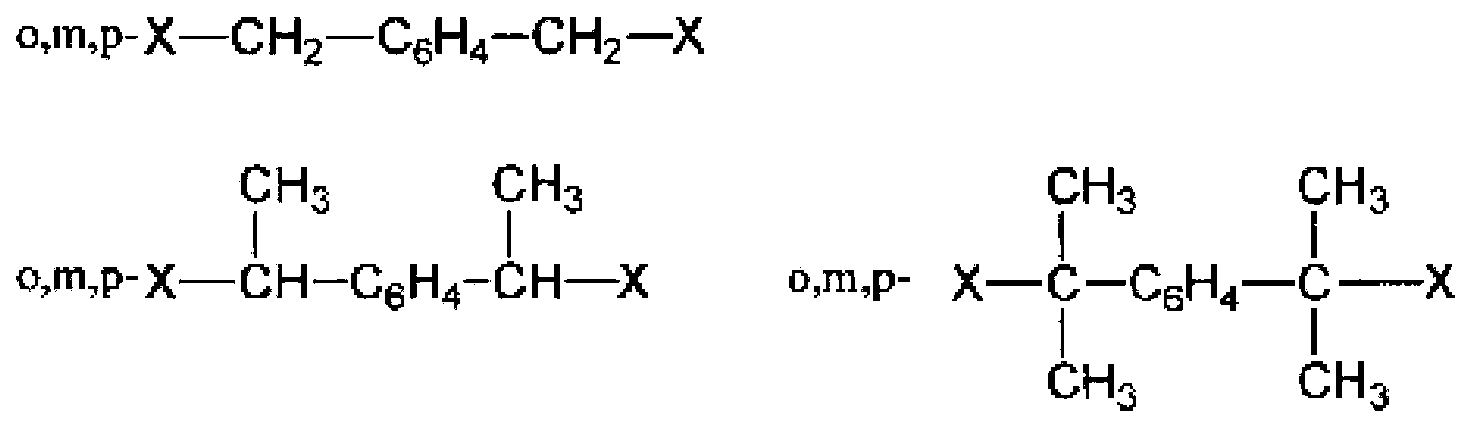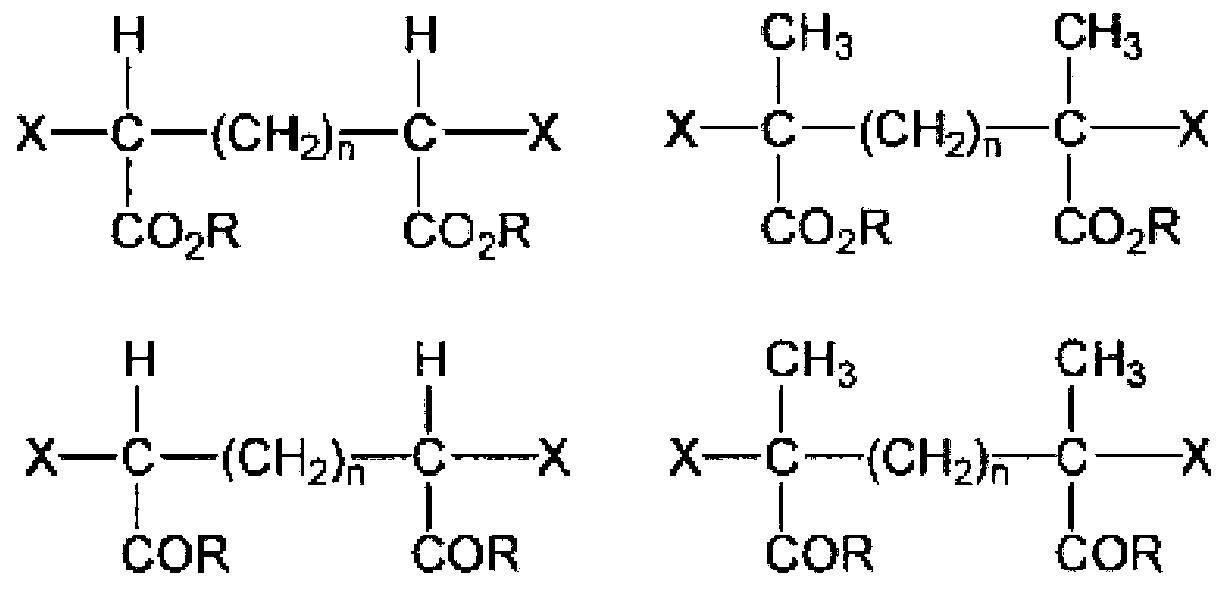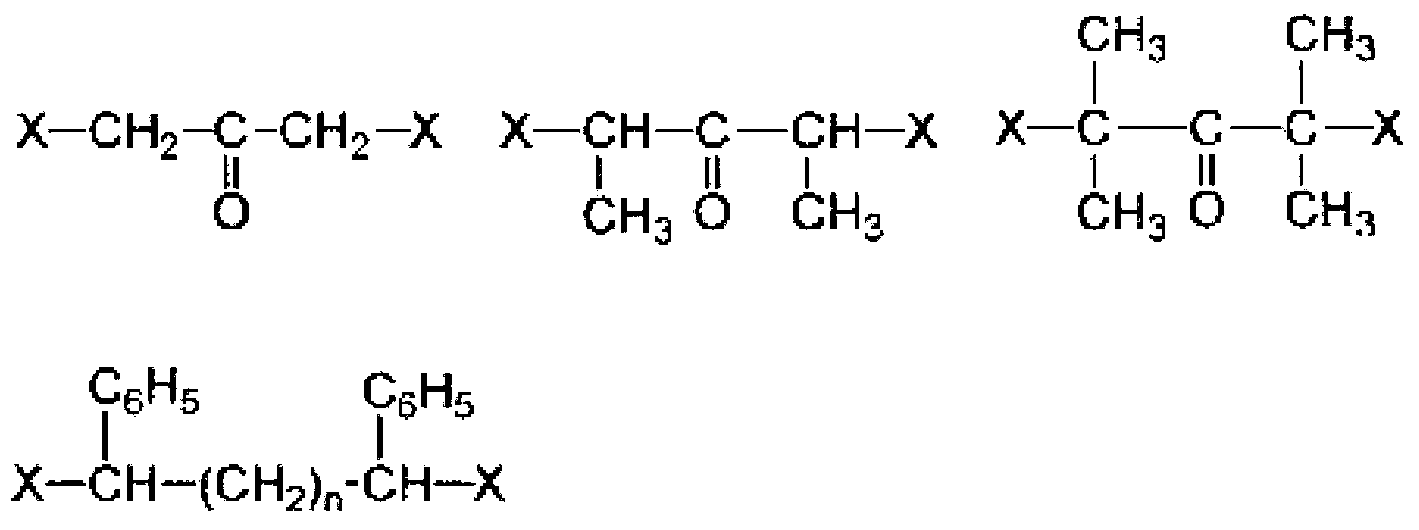Active energy ray-curable composition for optical material, cured product, and production methods for active energy ray-curable composition and cured product
A technology of active energy rays and curable compositions, applied in optics, optical components, instruments, etc., can solve the problems that cannot be used in optical materials that require high transparency, and achieve low viscosity and excellent active energy ray curability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0282] The present invention will be described in more detail by enumerating specific examples below, but the present invention is not limited to the following examples.
[0283] In the following examples, "number average molecular weight" and "molecular weight distribution (ratio of weight average molecular weight to number average molecular weight)" were calculated by the standard polystyrene conversion method using gel permeation chromatography (GPC). Here, a column packed with polystyrene cross-linked gel (Shodex GPC K-804 and K-802.5, manufactured by Showa Denko Co., Ltd.) was used as a GPC column, and chloroform was used as a GPC solvent.
[0284] In the following examples, "the number of (meth)acryloyl groups introduced per 1 polymer molecule" is given by 1 H-NMR analysis and calculation of number average molecular weight obtained by GPC. (in, 1 H-NMR was measured at 23° C. using ASX-400 manufactured by Bruker Co., Ltd., using deuterated chloroform as a solvent. )
...
Synthetic example 1)
[0309]
[0310] With cuprous bromide as the catalyst, pentamethyldiethylenetriamine as the ligand, 2,5-dibromodiethyl adipate as the initiator, and n-butyl acrylate as the monomer, in (n-butyl acrylate ester) / (diethyl-2,5-dibromoadipate) ratio of 80 to obtain bromine-terminated poly(n-butyl acrylate).
[0311] This polymer was dissolved in N,N-dimethylacetamide, potassium acrylate was added, and heated and stirred at 70°C in a nitrogen atmosphere. After distilling off N,N-dimethylacetamide in the mixture under reduced pressure, butyl acetate was added to the residue, and insoluble components were removed by filtration. Butyl acetate in the filtrate was distilled off under reduced pressure to obtain poly(n-butyl acrylate) having acryloyl groups at both terminals (polymer [P1]).
[0312] The number average molecular weight of the polymer [P1] was 12,000, the molecular weight distribution was 1.2, and the average number of terminal acryloyl groups was 1.8.
[0313] 4 g of a 5...
Synthetic example 2)
[0316]
[0317] With cuprous bromide as the catalyst, pentamethyldiethylenetriamine as the ligand, ethyl α-bromobutyrate as the initiator, and n-butyl acrylate as the monomer, in (n-butyl acrylate) / ( α-Bromobutyric acid ethyl ester) is polymerized under the condition of a ratio of 40 to obtain bromine-terminated poly(n-butyl acrylate).
[0318] This polymer was dissolved in N,N-dimethylacetamide, potassium acrylate was added, and heated and stirred at 70°C in a nitrogen atmosphere. After distilling off N,N-dimethylacetamide in the mixture under reduced pressure, butyl acetate was added to the residue, and insoluble components were removed by filtration. Butyl acetate in the filtrate was distilled off under reduced pressure to obtain poly(n-butyl acrylate) having an acryloyl group at one end.
[0319] To 800 g of the obtained polymer was added 4 g of a 50% aqueous hydrogen peroxide solution and stirred in air for about 10 minutes. After further stirring in air at 100°C for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| storage modulus | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com