Silicon phthalocyanine axially modified by aminoethyl phenoxyl and polyethylene glycol oligomer
A technology of aminoethylphenoxy and oligoethylene glycol, applied in chemical instruments and methods, botany equipment and methods, active ingredients of silicon compounds, etc., can solve the problem of lack of tumor tissue and cancer cell selectivity, clinical application Limitations, high skin phototoxicity and other issues, to achieve excellent amphiphilicity, improved tissue penetration ability, and clear structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
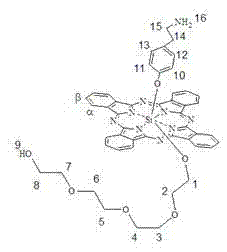
[0037] Synthesis and physical and chemical properties of bis[4-(2-aminoethyl)phenoxy] phthalocyanine silicon (structure shown in the following formula):
[0038]
[0039] Under the protection of nitrogen, add dichlorosilicon phthalocyanine (244.7mg, 0.4mmol), 4-(2-aminoethyl)phenol 1.2~2mmol (preferably 1.6mmol) and NaH to toluene or xylene or dioxane In the ring 20-50ml (preferably toluene, 30ml), reflux for 12-24 hours (preferably 18 hours). The solvent was removed by vacuum rotary evaporation, dissolved with 100ml of dichloromethane, centrifuged to remove insolubles, the dichloromethane solution was extracted with water (3×100ml), the organic layer was collected, and then extracted with dilute hydrochloric acid (0.1~0.5 mmol), and the water layer was collected. The water layer was neutralized with 1M sodium hydroxide, a blue precipitate was precipitated, centrifuged, washed with water, and dried in vacuum to obtain a blue product with a yield of 45%. The maximum absorption pe...
Embodiment 2
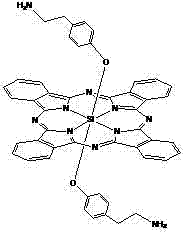
[0042] The structure is shown in the following formula: Synthesis and physicochemical properties of silicon phthalocyanine modified by axial aminoethylphenoxy and oligoethylene glycol:
[0043]
[0044] Add 40~100ml (preferably 60ml) triethylene glycol monomethyl to 1mmol of bis[4-(2-aminoethyl)phenoxy]phthalocyanine in toluene (or xylene or dioxane) solution Ether, add a catalytic amount of NaH and continue the reaction at 110°C. Monitor the reaction by TLC. After 5 hours, rotate the reaction mixture to at least the amount, add a small amount of DMF to dissolve, add a large amount of water, and precipitate a precipitate. Membrane filtration to remove the solvent and reaction materials And by-products, dry. Purify the crude product through a silica gel column, using ethyl acetate as the elution solvent, after the first band is completely eluted, use DMF as the elution solvent, collect the target elution components, evaporate at least the amount of solvent, filter, and spin. Afte...
Embodiment 3
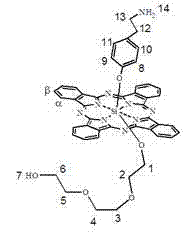
[0047] Synthesis and physicochemical properties of silicon phthalocyanine modified with axial aminoethylphenoxy and oligoethylene glycol with the structure shown in the following formula:
[0048]
[0049] Add 40~100ml (preferably 60ml) of triethylene glycol to 1mmol of bis[4-(2-aminoethyl)phenoxy]phthalocyanine in toluene (or xylene or dioxane) solution, and add After a catalytic amount of NaH, the reaction was continued at 110°C, and the reaction was monitored by TLC. After 5 hours, the reaction mixture was rotated to at least the amount, and a small amount of DMF was added to dissolve, and a large amount of water was added to precipitate a precipitate. Membrane filtration to remove solvent, reaction materials and by-products ,dry. The crude product was purified by a silica gel column, using ethyl acetate as the eluting solvent, and ethyl acetate as the eluting solvent through the silica gel column. After the first band was completely eluted, a mixed solvent of DMF and triethyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com