Doxycycline hydrochloride dry suspension and preparation method thereof
A technology of doxycycline hydrochloride and dry suspension, which is applied in the directions of tetracycline active ingredients, pharmaceutical formulations, and non-active ingredients medical preparations, etc., can solve the problems of poor absorption and dispersion stability, difficult to swallow tablets, and poor palatability problems, to achieve the effect of improving palatability, fast absorption, and large distribution area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
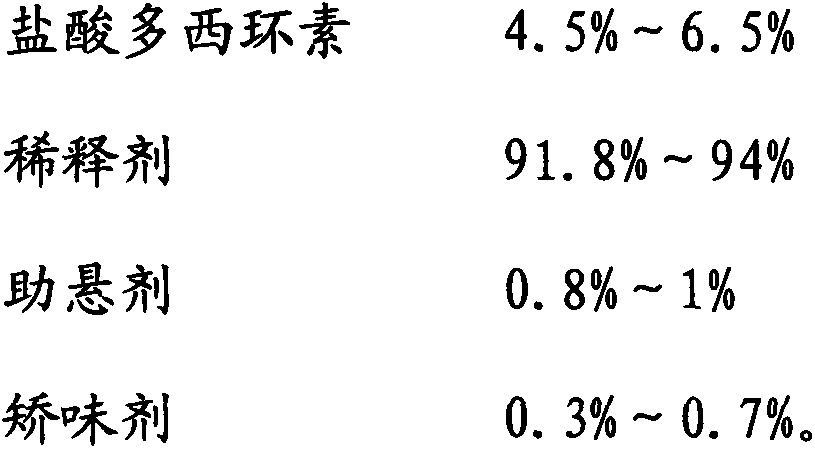
[0020] Doxycycline hydrochloride dry suspension, 1000 mg per bag, the formula contains: 50 mg of doxycycline hydrochloride (crude drug, the same below), 935 mg of diluent, 10 mg of suspending agent, and 5 mg of flavoring agent.
[0021] Wherein, the diluent is glucose, the suspending agent is hydroxypropyl cellulose, and the corrective agent is banana essence.
[0022] Preparation method: dry mix doxycycline hydrochloride, diluent, suspending agent and flavoring agent for 10 minutes, pulverize, pass through a 120-mesh sieve, and fill to obtain.
[0023] The doxycycline hydrochloride dry suspension prepared in this example was checked for the sedimentation volume ratio according to the relevant regulations under the dry suspension inspection item in the appendix II of the Chinese Pharmacopoeia 2010 edition, and the result was 0.98. At the same time, the dispersion stability when taken is 90% higher than that of tablets and capsules.
Embodiment 2
[0025] Doxycycline hydrochloride dry suspension, calculated as 1000 mg per bag, the formula contains: 45 mg of doxycycline hydrochloride, 940 mg of diluent, 8 mg of suspending agent, and 7 mg of flavoring agent.
[0026] Wherein, the diluent is sucrose, the suspending agent is sodium carboxymethyl starch 6 mg, xanthan gum 2 mg, and the flavoring agent is fresh milk essence.
[0027] Preparation method: dry mix doxycycline hydrochloride, diluent, suspending agent and flavoring agent for 15 minutes, pulverize, pass through a 100-mesh sieve, and fill to obtain.
[0028] The sedimentation volume ratio is 0.97, and the dispersion stability when taken is 70% higher than that of tablets and capsules.
Embodiment 3
[0030] Doxycycline hydrochloride dry suspension, 2000 mg per bag, the formula contains: 130 mg of doxycycline hydrochloride, 1836 mg of diluent, 20 mg of suspending agent, and 14 mg of flavoring agent.
[0031] Wherein, the diluent is xylitol, the suspending agent is hydroxypropyl cellulose 10 mg, xanthan gum 10 mg, and the corrective agent is almond essence.
[0032] The preparation method is the same as in Example 1.
[0033] The sedimentation volume ratio is 0.97, and the dispersion stability when taken is 75% higher than that of tablets and capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com