Polymer material containing large-volume branching alkoxy side chain, as well as preparation method and application of polymer material
A technology of polymer materials and branched alkanes, which is applied in the application field of solar cells, can solve problems such as side chain structure photoelectric performance reaction, achieve excellent solubility, excellent electrical properties, and simplify the processing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Synthetic route to BDT tinned monomers containing bulky branched alkoxy side chains
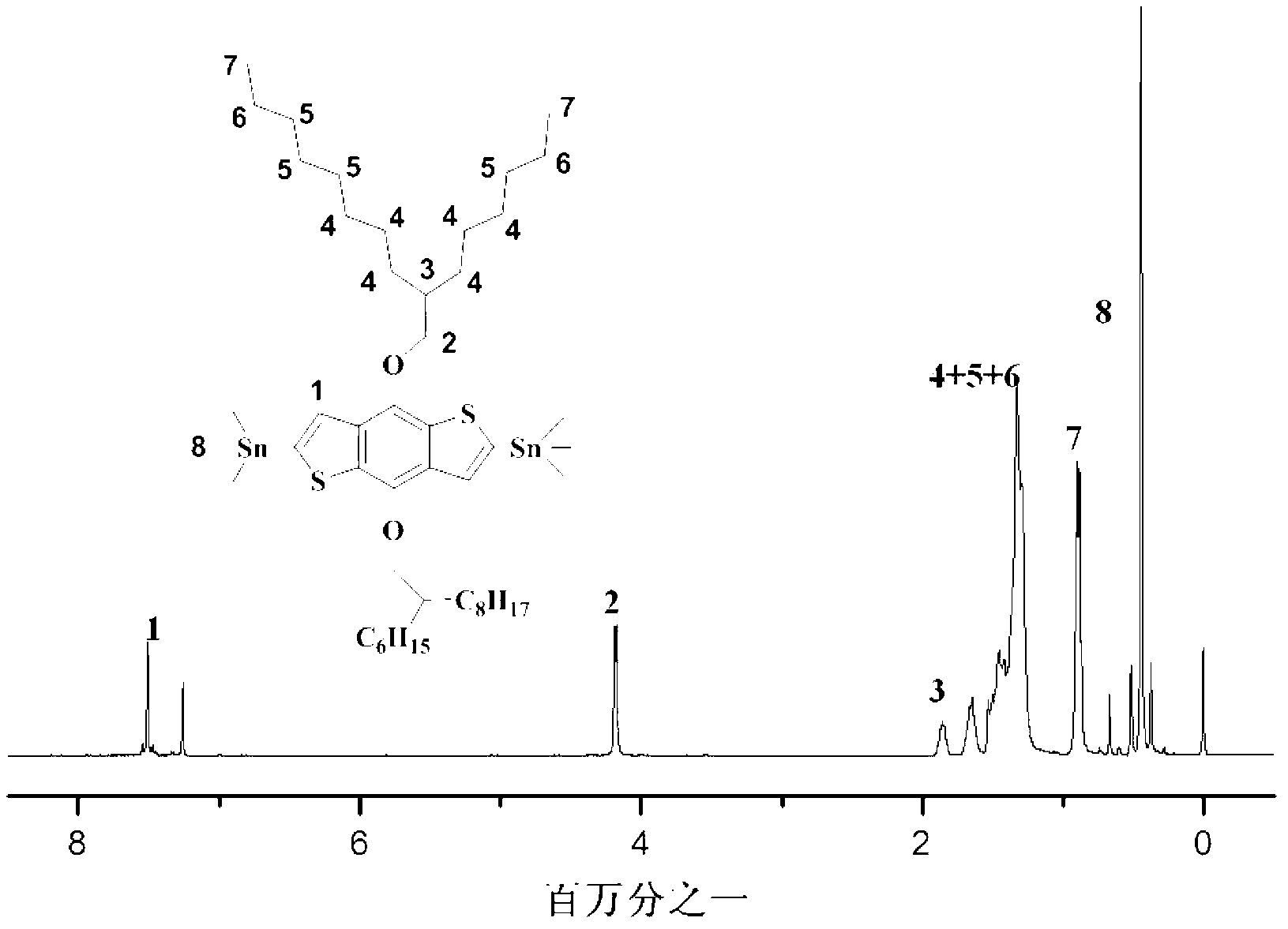
[0029]BDT tin monomer is one of the pre-polymerized monomers of D-A type narrow-band polymers, which provides 4,8-dialkoxybenzobithiazole as the donor unit part for the polymer structure. This series of BDT tinned monomers containing bulky branched alkoxy side chains was synthesized and applied for the first time by us, and they played a key role in improving the solubility of polymers. The following is the introduction of the synthesis route and specific synthesis method of this series of monomers.
[0030] The following is 2,6-bis(trimethyltinyl)-4,8-bis(2-hexyldecyloxy)benzo[1,2-b:4,5-b, one of the series of monomers '] dithiophene monomer (B HD DT-Sn), the synthesis process of other monomers in this series is consistent with this.
[0031]
example 1
[0032] Example 1.BDT tinned monomer (B HD DT-Sn) synthetic route
[0033] Preparation of mesylated 2-hexyldecane (2-hexyldecylmethanesulfonate, 1):
[0034] Add 12.12g (0.05mol) of 2-hexyldecanol and 250mL of dichloromethane into a 500mL two-neck flask, stir electromagnetically at room temperature, then add 4.3mL (0.054mol) of methanesulfonyl chloride dropwise, stir at room temperature for 1 hour, then add dropwise 7.6 mL (0.054 mol) of triethylamine was added, and stirring was continued for one hour at room temperature. The solvent was then removed by rotary evaporation, and the resulting oily residue was extracted with water and ether, MgSO 4 After drying, it was filtered, spin-dried, and purified by a silica gel column to obtain 11.22 g of a yellow transparent oil with a yield of 75%. 1 H NMR (400MHz, CDCl 3 ):d=4.09(d,2H),2.96(s,3H),1.68–1.66(quintet,1H),1.42-1.25(m,22H),0.86ppm(t,6H,). 13 C NMR (100MHz, CDCl 3 ):d=72.44,37.68,36.99,31.76,30.56,29.72,29.38,26.42,22.5...
Embodiment 2
[0041]
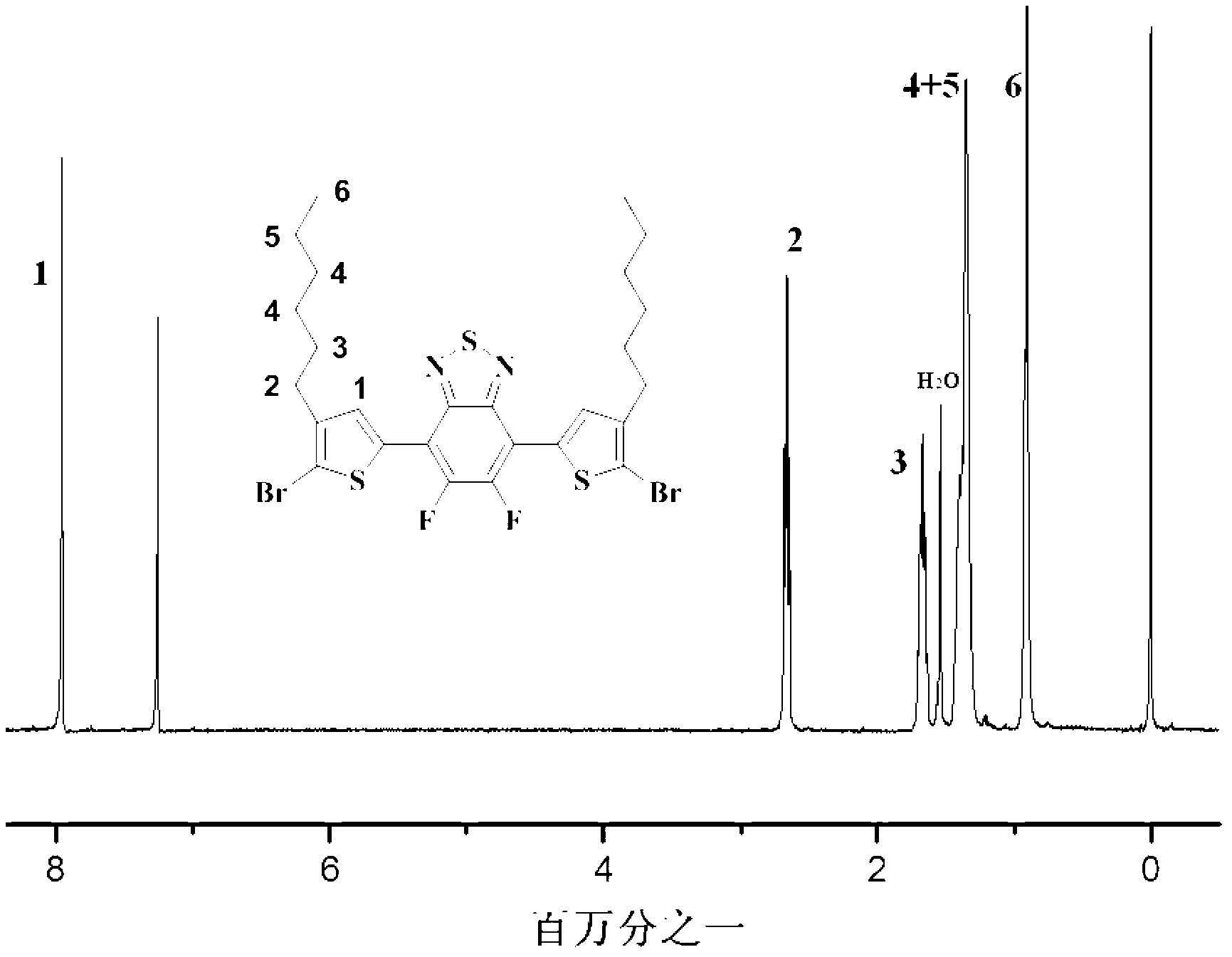
[0042] Dibromo-DTBT ff Monomer Synthetic Route
[0043] Dibromo-DTBT ff The monomer is another pre-polymerized monomer of the D-A type narrow-band polymer, which provides the polymer structure with 5,6-difluoro-4,7-dialkylthiophene-2,1,3-benzothiazole monomer ( DTBT ff ), as part of the acceptor unit. This series of dibrominated DTBTs with alkyl side chains of different lengths ff The monomers were synthesized and applied to this polymer structure for the first time by us, and they also played a certain role in improving the solubility of the polymer. The following is the introduction of the synthesis route and specific synthesis method of this series of monomers: Fluoro-2,1,3-benzothiazole monomer (B HD DT-Sn), the synthesis process of other monomers in this series is consistent with this.
[0044]
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com