Sulphated heparan sulfated for inhibiting cell proliferation
A technology of heparan sulfate and sulfate esterification, which is used in medical preparations containing active ingredients, organic active ingredients, antitumor drugs, etc., can solve the difficulty of preparing high-purity HS, has not been widely used, and the effect of natural HS is not significant And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] After freeze-drying the HS prepared by the reference patent "A Method for Purifying Heparan Sulfate from Heparin By-products", take 0.5g and dissolve it in 30mL of anhydrous DMF, fully swell, add 2.5g (CH 3 ) 3 N·SO 3 , The reaction was stirred at 80°C for 3h. After cooling to room temperature, clear the supernatant, dissolve the precipitate in distilled water, dialyze, freeze-dry, and dissolve it with 10% sodium hydroxide to prepare a 100 mg / mL solution, precipitate it with ethanol with a final concentration of 80%, and dry it. Sulfated HS is produced.
Embodiment 2
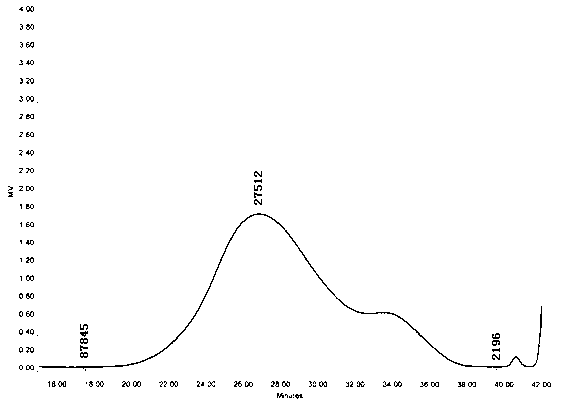
[0021] The molecular weight of the sulfated HS was determined by high performance liquid gel exclusion chromatography. The mobile phase is a mixed solution of 0.1mol / L ammonium acetate and 0.02% sodium azide, the molecular weight reference substance is heparin sodium (NIBSC code 07 / 324), and the tandem chromatographic column TSK Guard column (7.8mm×30cm), TSK SW xl4000 ( 7.8mm×30cm) and TSK SW xl3000 (7.8mm×30cm), column temperature 30°C, elution flow rate 0.6ml / min, differential refraction detector, temperature 30°C. GPC software draws the standard curve of heparin sodium reference substance molecular weight and retention time as: LogM w =1.09e +001 -5.06e -001 T+1.41e -002 T 2 -1.55e -004 T 3 (R 2 =9999). GPC software calculates the molecular weight M of sulfated HS w =27521Da, the molecular weight distribution is 2196~87845Da, the components with molecular weight greater than 24000Da account for 49.55%, and the components with molecular weight less than 8000Da acc...
Embodiment 3
[0023] Take a 96-well cell culture plate and add about 5×10 3Individual hepatoma cells (HepG2), in 5% CO 2 Cultivate in the incubator at 37°C for 12 hours, add DMEM medium containing 1 mg / mL and 0.1 mg / mL sulfated HS respectively; 2 Cultivate in an incubator for 48 hours, add 10 μL MTT to each well, incubate for 4 hours, absorb the medium, add 150 μL DMSO, shake well, and detect at 490 nm on an enzyme-linked immunosorbent analyzer, the absorbance value of the sample is A 1 , the absorbance of the blank is A 2 , inhibition rate (%)= . The inhibition rates of HepG2 by sulfated HS at concentrations of 1mg / mL and 0.1mg / mL were 47.12% and 41.53%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com