Improved adenovirus vector system, preparation and application of its virus particle
A technology of adenovirus and recombinant adenovirus, applied in virus/bacteriophage, application, botanical equipment and methods, etc., can solve the problems of operator chemical hazards, long time-consuming, low success rate, etc., and avoid the use of harmful chemical reagents , Operation time is shortened, and reagent cost is reduced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The amplified MCS-IRES-GFP fragment is as follows:
[0056] Design and synthesize 2 PCR primers, the sequences are as follows:
[0057] 5'-GGGGACAAGTTTGTACAAAAAAGCAGGCTGCGGCCGCTTTCTAGACTCAGATCTCGAGCT-3' (SEQ ID NO. 1);
[0058] 5' - GGGGACCACTTTGTACAAGAAAGCTGGGTCTTACTTGTACAGCTCGTCCAT-3' (SEQ ID NO. 2).
[0059] The pIRES-EGFP vector (purchased from Clontech) was used as a template, and the above two oligonucleotide chains were used as primers to amplify to obtain the DNA fragment of MCS-IRES-GFP. The fragment amplified from the pIRES-EGFP vector contains multiple cloning sites NotI, XbaI, BglII, XhoI, SacI, EcoRI, PstI, SalI, SacII, SmaI, and BamHI.
[0060] 1) The volume of each component in the PCR system
[0061] Component
volume
Ultra-pure water
37.8μl
10XPCR buffer
5μl
3μl
dNTPs (25mM)
0.4μl
Upstream primer (10μM)
1μl
Downstream primer (10μM)
1μl
Taq enzy...
Embodiment 2
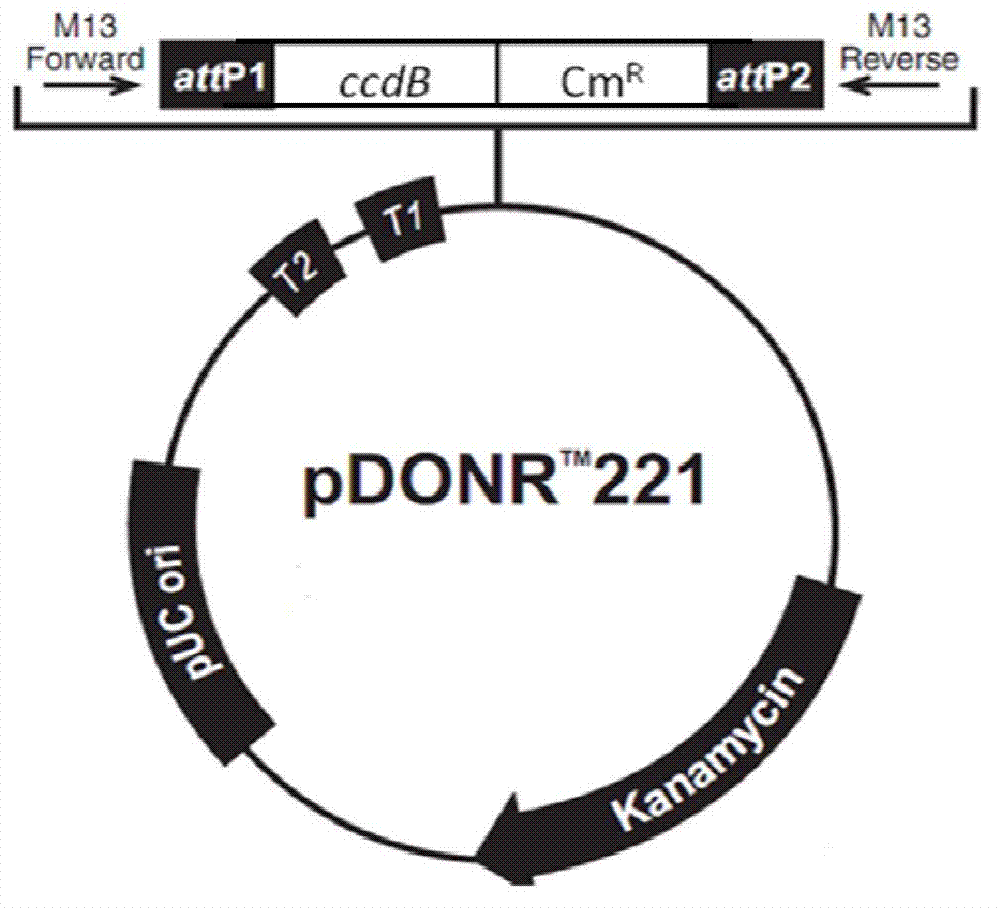
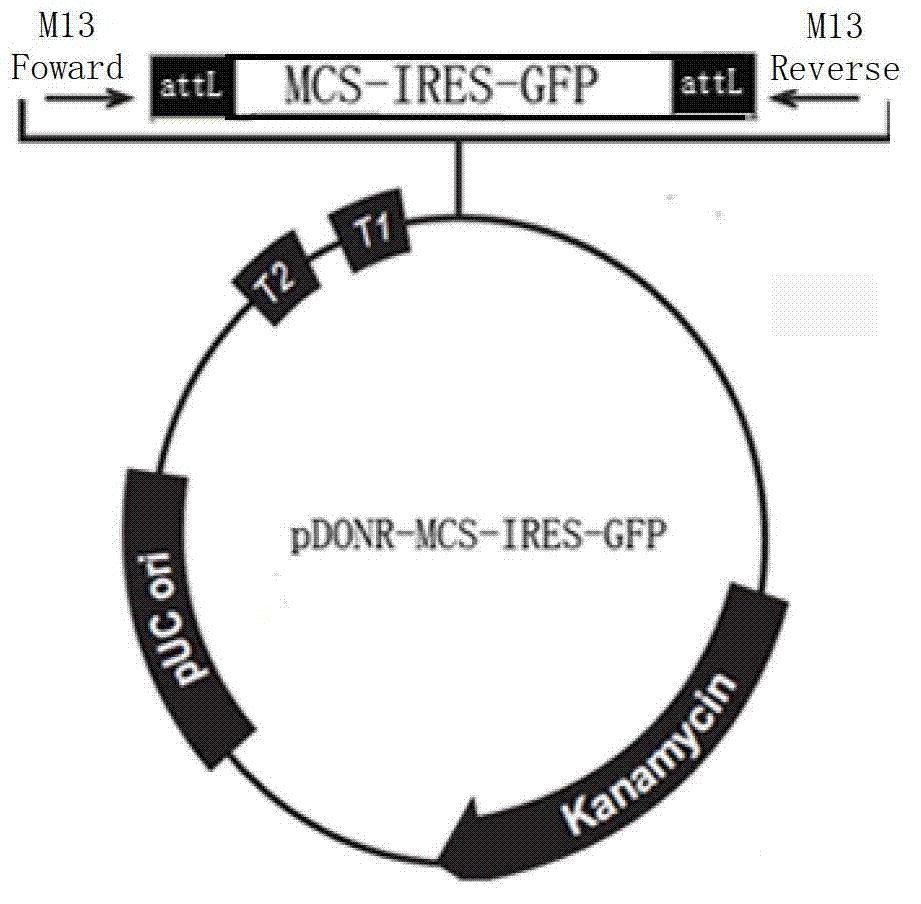
[0068] The pDONR221 vector (Invitrogen, figure 1 ) into a pDONR-MCS-IRES-GFP vector, including purifying the above-mentioned amplified MCS-IRES-GFP fragment, performing BP recombination with pDONR221, and the obtained vector was named pDONR-MCS-IRES-GFP (see figure 2 ). details as follows:
[0069] Use Invitrogen's BP recombination system (Invitrogen's BP recombination kit) to recombine the target fragment into the vector pDONR221. Prepare the BP recombination reaction system according to the following table:
[0070] PCR product
2μl
pDONR221 plasmid
1μl
BP clonaseⅡenzyme mix
2μl
TE buffer (pH8.0)
5μl
[0071] React at 25°C for 1 hour.
[0072] Add 1 μl of proteinase K and react at 37°C for 10 minutes.
[0073] Take 5 μl of the recombination reaction solution to transform 50 μl of Escherichia coli DH5α competent cells.
[0074] Positive clones were screened and verified by sequencing, and the correct BP recombinant pl...
Embodiment 3
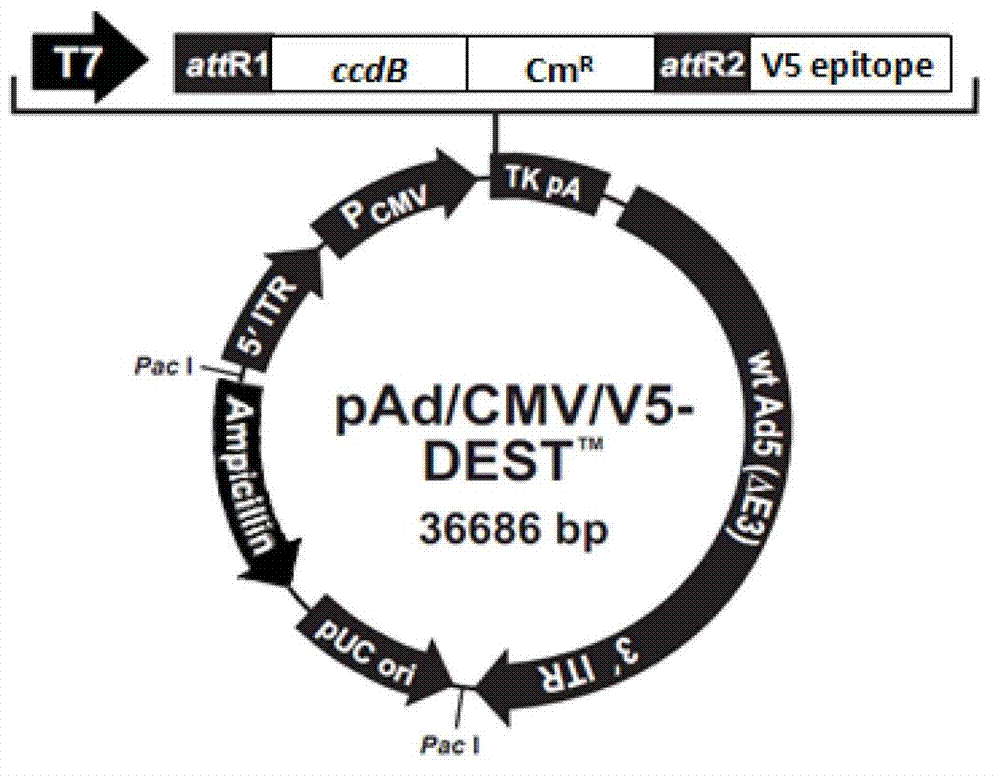
[0077] The pDONR-MCS-IRES-GFP vector prepared in Example 2 and the pAd / CMV / V5-DEST vector (purchased from Invitrogen, image 3 ) for LR recombination to obtain the final recombination vector pAd / MCS-IRES-GFP. details as follows:
[0078] LR recombination was performed using Invitrogen's LR recombination system (Invitrogen's LR recombination kit). Prepare the LR recombination reaction system according to the following table:
[0079] BP recombinant plasmid
1μl
pAd CMV / V5-DEST
1μl
LR clonaseⅡenzyme mix
2μl
TE buffer (pH8.0)
6μl
[0080] React at 25°C for 1 hour.
[0081] Add 1 μl of proteinase K and react at 37°C for 10 minutes.
[0082] Take 5 μl of the recombination reaction solution to transform 50 μl of DH5α competent cells.
[0083] Positive clones were screened and verified by sequencing, and the correct LR recombinant plasmids verified by sequencing were retained.
[0084] The schematic diagram of the vector const...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com