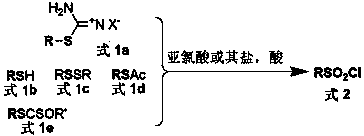
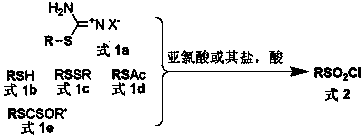
General preparation method of sulfonyl chloride
A technology of sulfonyl chloride and oxidative chlorination, which is applied to the preparation of sulfonic acid, the preparation of organic compounds, chemical instruments and methods, etc., and can solve problems such as operational hazards, generation of acidic or toxic by-products, excessive use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Benzylsulfonyl chloride 2a preparation of
[0058] Thiourea (0.387 g, 5 mmol) and benzyl chloride (0.633 g, 5 mmol) were dissolved in 5 mL of ethanol. After reflux for 30 min, the solvent was removed under reduced pressure to obtain a white solid. The white solid was slowly added dropwise to NaClO 2 (1.61 g, 15 mmol, 85% purity), in a mixed system of concentrated HCl (3 mL) and MeCN (10 mL). During the feeding process, use a water bath to control the temperature in the reaction system at 10-20 oC between. After the addition, continue to stir the reaction for 30 min, remove acetonitrile under reduced pressure at low temperature, add 25 mL of water, filter the solid in the system, and dry to obtain the product colorless crystal benzylsulfonyl chloride 3a , melting point 90-91°C, 0.781 g, yield 82%. 1 H NMR (400 MHz, CDCl 3 ) δ: 7.61~7.35 (m, 4H), 5.12 (s, 2H).
Embodiment 2
[0060] p-methylbenzylsulfonyl chloride 2b preparation of
[0061] According to the method described in Example 1, using p-methylbenzyl chloride and thiourea as raw materials, p-methylbenzylsulfonyl chloride was obtained as colorless crystals, melting point 86-88°C, 0.665 g, yield 65%. 1 H NMR (400 MHz, CDCl 3 ) δ: 7.38~7.26 (m, 4H), 4.83 (s, 2H), 2.39 (s, 3H).
Embodiment 3
[0063] 4-chlorobenzylsulfonyl chloride 2c preparation of
[0064] According to the method described in Example 1, using p-chlorobenzyl chloride and thiourea as raw materials, p-chlorobenzylsulfonyl chloride was obtained as colorless crystals with a melting point of 90-92°C, 1.080 g, and a yield of 96%. 1 H NMR (400 MHz, CDCl 3 ) δ: 7.46~7.42 (m, 4H), 4.83 (s, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com