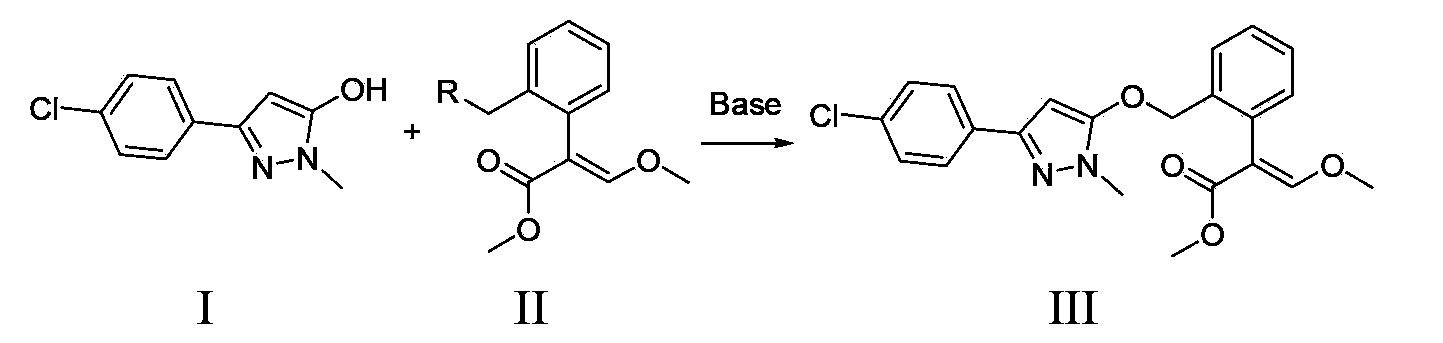
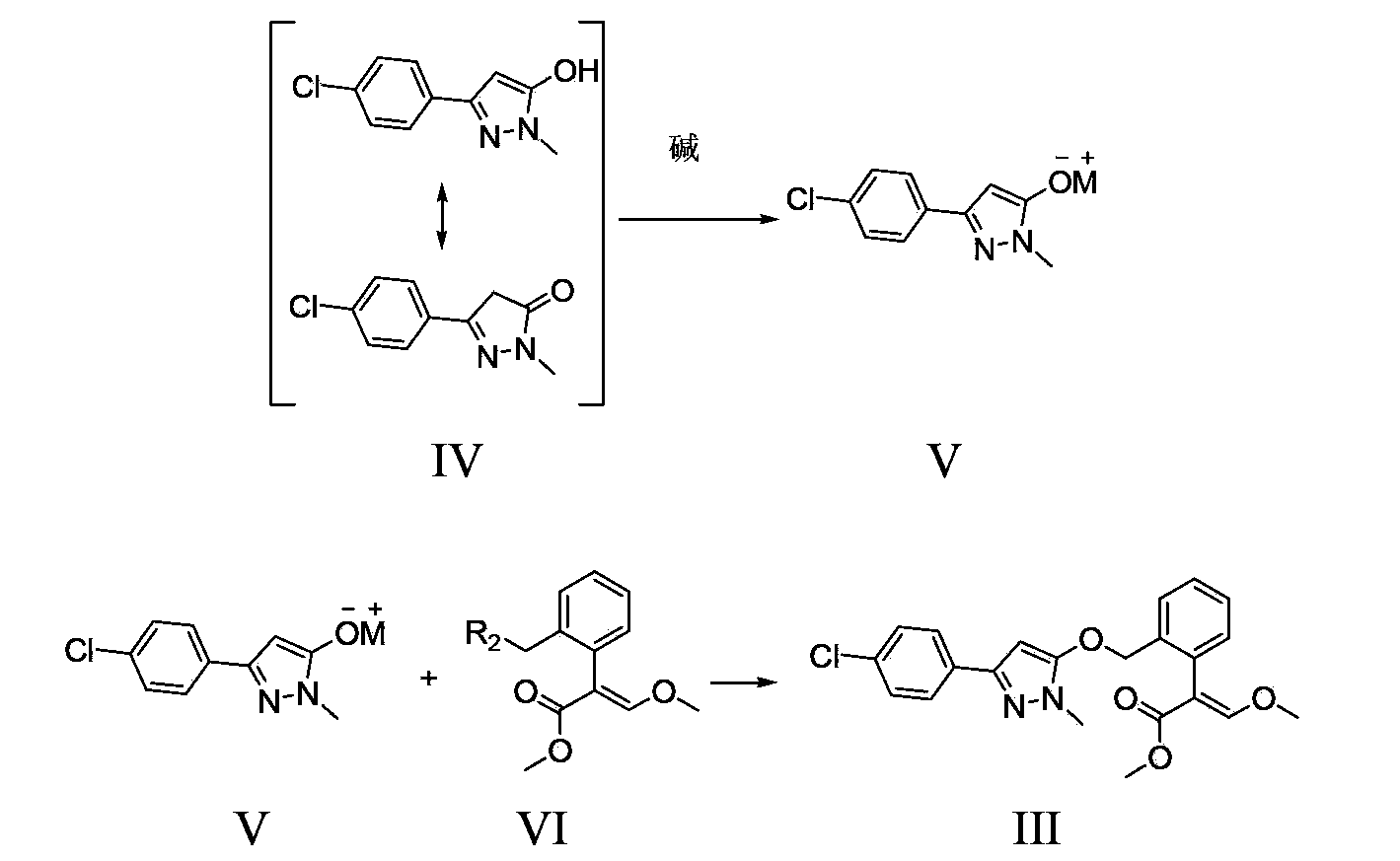
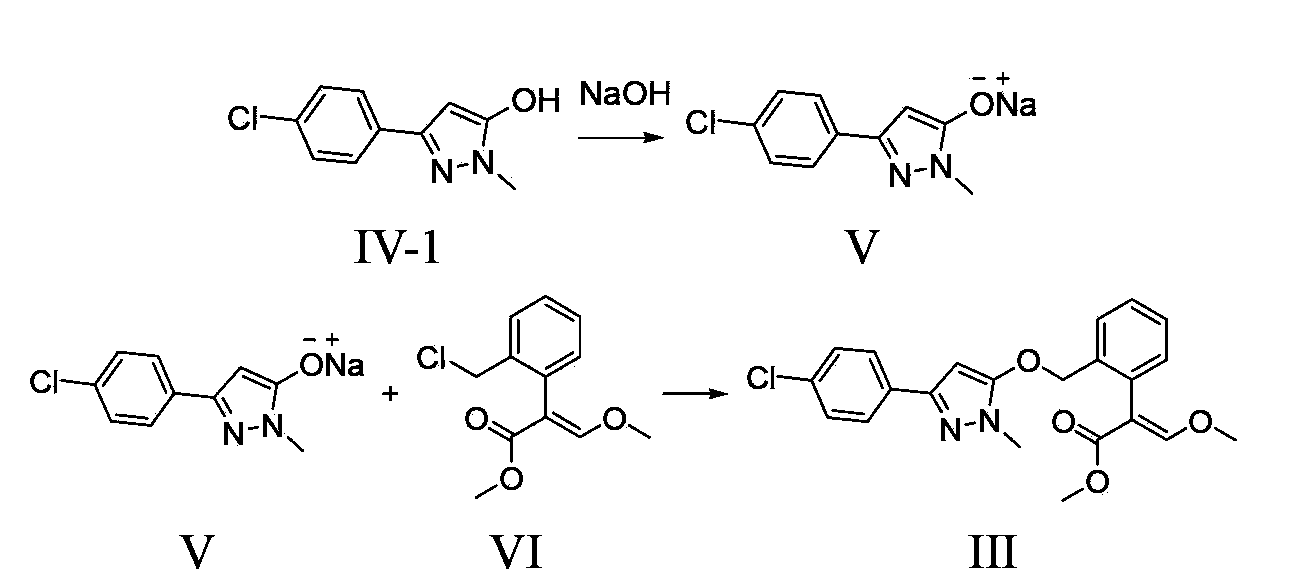
Method for preparation of pyraoxystrobin by salt forming method
The technology of pyraclostrobin and salt-forming method is applied in the field of preparing pyraclostrobin by salt-forming method, which can solve the problems of increased carbon alkylation by-products, hydrogen generation, reduction of oxyalkylation products, etc., and achieves easy industrial production, The method is simple and easy to implement, and the reaction conditions are mild.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] 1). Add 11.7 g (54.7 mmol) of 3-(4-chlorophenyl)-1-methyl-1H-5-pyrazolol and 100.0 g of toluene in sequence to a 250 ml reaction bottle equipped with a water separator 2.2 g (54.5 mmol) of sodium hydroxide was added, and the temperature was raised to reflux for 8 hours, while the generated water was separated. After the reaction was completed, toluene was recovered by distillation, and the residue in the reaction bottle was white 3-(4-chlorophenyl)-1-methyl-1H-5-pyrazole sodium salt solid. Add 30.0 g of DMF and 12.4 g (50.0 mmol) of methyl (E)-2-(2-chloromethylphenyl)-3-methoxyacrylate into the reaction flask, and raise the temperature to between 90°C and 95°C The heat preservation reaction was carried out for 6 hours, and the reaction was completed.
[0041] 2). Recover DMF by distillation under reduced pressure. Add 80.0 grams of toluene and 80.0 grams of water to the reaction bottle, extract, stand still, and layer to get the upper pyraclostrobin toluen...
Embodiment 2
[0043]
[0044] 1). Add 11.7 g (54.7 mmol) of 3-(4-chlorophenyl)-1-methyl-1H-5-pyrazolol and 10 g of DMSO to a 250 ml reaction bottle equipped with a water separator and 100.0 g of n-heptane, heat up and reflux for water separation for 2 hours, cool down to room temperature, add 3.0 g of sodium methoxide (55.0 mmol), heat up and reflux to separate methanol for 12 hours, then distill to recover n-heptane, and the residue in the reaction bottle is white 3 -(4-Chlorophenyl)-1-methyl-1H-5-pyrazolol sodium salt solid. Add 30.0 g of DMSO and 15.5 g (49.9 mmol) of methyl (E)-2-(2-bromomethylphenyl)-3-methoxyacrylate into the reaction flask, and raise the temperature to between 65°C and 70°C The heat preservation reaction was carried out for 5 hours, and the reaction was completed.
[0045] 2).Recover DMSO by distillation under reduced pressure. Add 80.0 grams of ethyl acetate and 80.0 grams of water to the reaction bottle, extract, stand still, and separate layers to obtain the ...
Embodiment 3
[0047]
[0048] 1). Add 5-(4-chlorophenyl)-2,4-dihydro-2-methyl-3H-3-pyrazolone 23.4 g (109.1 mmol) and toluene 50.0 grams, 50.0 grams of water, and 6.8 grams (109.2 mmol) of potassium hydroxide, heated and refluxed for 8 hours, distilled toluene and water, and the residue was pink 3-(4-chlorophenyl)-1-methyl-1H - 5-Pyrazolol sodium salt solid, add 30.0 g of DMF to dissolve and set aside. Add 30.0 g of DMF and 24.8 g (100.0 mmol) of methyl (E)-2-(2-chloromethylphenyl)-3-methoxyacrylate in sequence to a 500 ml reaction flask, and raise the temperature to 90°C-95 Between ℃, DMF solution of sodium salt was added dropwise, added dropwise for 2 hours, and kept at 90°C-95°C for 3 hours, and the reaction ended.
[0049] 2). Recover DMF by distillation under reduced pressure. Add 150.0 grams of dichloromethane and 100.0 grams of water into the reaction bottle, extract, stand still, and separate layers to obtain the lower pyraclostrobin dichloromethane solution, distill and recove...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com