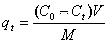Preparation method of graphene oxide and magnetic mesoporous silica composite material capable of adsorbing pollutants in water
A technology of mesoporous silica and composite materials, which is applied in the fields of adsorption water/sewage treatment, alkali metal oxides/hydroxides, chemical instruments and methods, etc. Low cost of raw materials and efficient adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
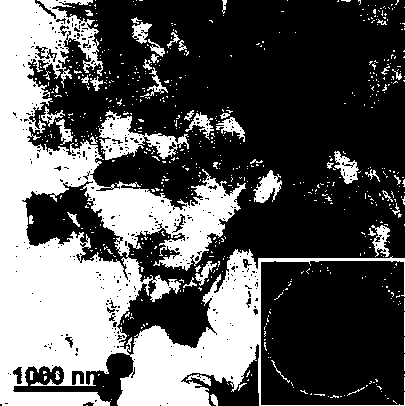
[0033] Example 1. 0.86 g FeCl 3 ·6H 2 O and 2.0 g sodium acetate are dissolved in 30 mL appropriate amount of ethylene glycol, quickly mechanically stirred to dissolve, put into a 50mL capacity PTFE lining, plus a stainless steel jacket, in a muffle furnace or blast drying box, Set the temperature to 200°C, and the reaction proceeded for 8 hours. After natural cooling, it was washed three times with ethanol and water to obtain inorganic magnetic particles with an average particle size of 330 nm. Wash it ultrasonically for 15 minutes in a hydrochloric acid aqueous solution with a concentration of 1M, and wash until neutral.
[0034] Disperse 150 mg of the seeds obtained after washing in a mixed solvent of deionized water and absolute ethanol (v / v=30 / 70), add an appropriate amount of ammonia water, adjust the pH of the system between 9.0-9.5, and add under ultrasonic mechanical stirring 80.4 μL of tetraethyl orthosilicate, the reaction was mechanically stirred for 24 h at room t...
Embodiment 2
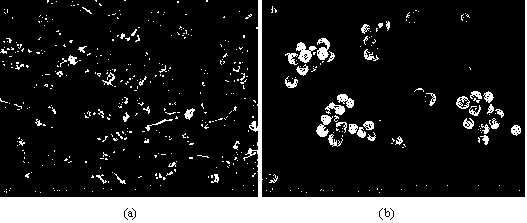
[0038] Example 2. The same method is used to prepare silica magnetic composite microspheres. By increasing the amount of sodium acetate to 3.0 g, magnetic microspheres with a particle size of 220 nm can be prepared; using the same silicon coating process, in different proportions of deionized water and In a mixed solvent of water and ethanol (v / v=10 / 90), silica magnetic microspheres with a particle size of about 250 nm were prepared; as seeds, the same mesoporous silica coating process was used, and orthosilicic acid was changed. The amount of tetraethyl and hexadecyltriethoxysilane can be used to prepare mesoporous silica magnetic microspheres with an average particle size of about 310 nm. Using the same basic method of PEI modification and increasing the amount of PEI to 30 mg, more amine groups can be introduced on the surface of the mesoporous silica magnetic microspheres. On the one hand, it can strengthen the binding efficiency with graphene oxide, and it can also improv...
Embodiment 3
[0039] Example 3. The graphene oxide mesoporous silica magnetic composite material obtained in the above Examples 1 and 2 was used for the adsorption experiment of Pb(II) and HA. The adsorption experiment is carried out at 25℃. Generally, 1.0 mg of the composite material is dispersed in a 10 mL liquid glass tube, and different initial concentrations of Pb(II) are configured, such as 5,10,20,50,80,100 mg / L, adjust the pH of the solution close to neutral, shake and combine at 150 rpm at room temperature for 24 h. When the adsorption reaches equilibrium, place the sample on the magnet for full separation. Use ICP-AES to test the absorbance of the remaining heavy metal ions in the solution, using the above formula 1. To calculate the adsorption capacity. See attached for typical adsorption isotherms Figure 4 .
[0040] The composite material can also synergistically adsorb humic acid and heavy metal ions. We used HA and without HA to investigate the adsorption characteristics of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| The average thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com