C genotype adefovir dipivoxil drug-resistant HBV (Hepatitis B Virus) stable replication and expression cell line
An adefovir dipivoxil and cell line technology, which is applied in the field of stable virus replication and expression cell lines, can solve the problem that it is difficult to fully reflect the phenotypic characteristics of drug-resistant strains directly isolated from patient serum, and achieve high application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
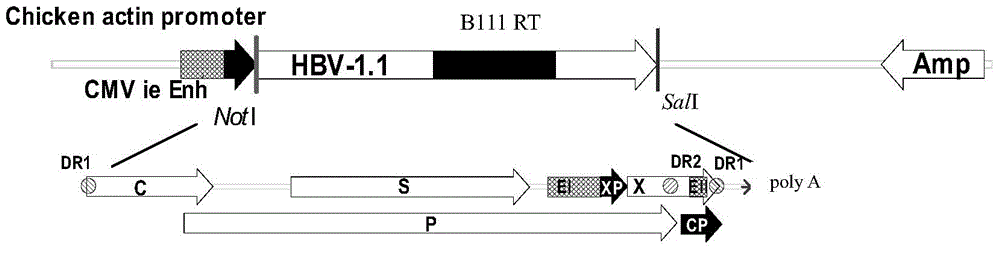
[0057] Example 1 Construction of 1.1 times longer HBV expression vector
[0058] The patient has been taking adefovir dipivoxil antiviral treatment for a long time, the viral load has been negative (figure 1 ). Five primers were synthesized inside the HBV sequence for full-length sequencing identification, and the results were completely consistent with the original sequence. The reverse transcriptase region contained a typical adefovir dipivoxil resistance mutation site rtA181V+rtN236T.
Embodiment 2
[0059] Example 2 Stable cell line screening
[0060] (1) Plasmid DNA transfection and cell clone screening Extract the transfection-grade recombinant plasmid pTriEx-1.1-HBV B111, and quantify the concentration with an ultraviolet spectrophotometer. At 37°C, containing 5% CO 2 HepG2 cells were cultured in complete DMEM medium with 10% FBS. Before transfection, the cells were inoculated into 24-well plates and cultured overnight. When the cell fusion reached 80%, the DMEM without serum and antibiotics was replaced, and the DNA was introduced with FuGENE HD liposomes. The specific method was carried out according to the operation manual. 5h after transfection, add FBS to 10%. Set untransfected wells as negative controls. Add G418 (final concentration 600 μg / ml) to the cell culture dish for 48 hours for screening, change the medium every 2 days, and replace it with G418 (300 μg / ml) at a maintenance concentration after the cells in the control group are completely dead to contin...
Embodiment 3
[0062] Example 3 Construction of the relevant detection of the resulting cell line
[0063] 1. Expression of major HBV antigens
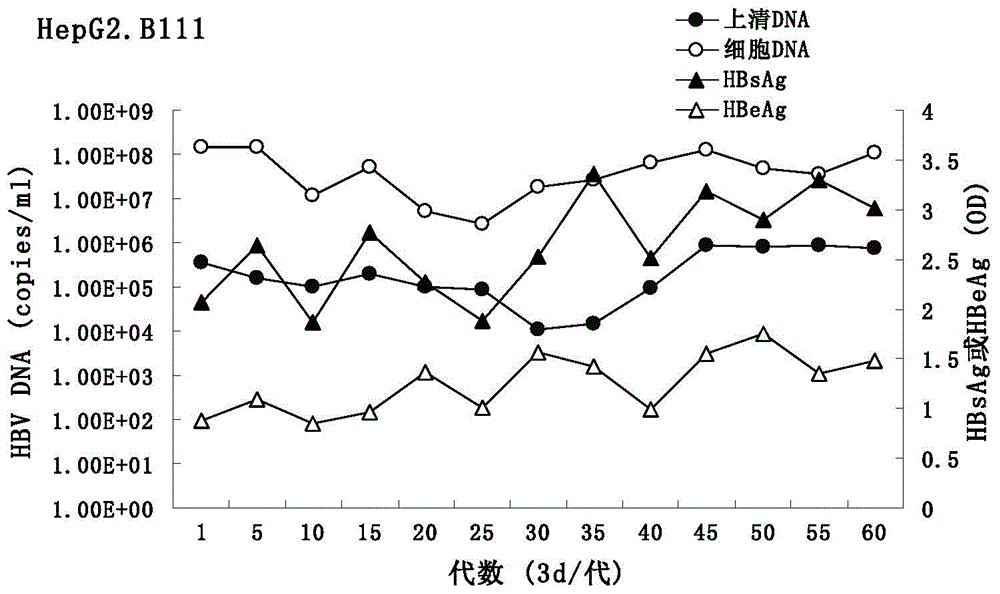
[0064] (1) ELISA detection Collect the cell culture supernatant of the HepG2.B111 cell line at passages 1, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, and 60 for ELISA (double antibody sandwich method) detection For the level of HBsAg and HBeAg, the wavelength is set to 450nm. With the increase of cell passage number, the expression of antigen gradually increased and tended to be stable, the average OD value of HBsAg was 2.64, HBeAg was 1.25 ( image 3 ), the corresponding HBsAg and HBeAg of HepG2.2.15 cells were 0.93 and 3.01 ( Figure 4 ).
[0065] (2) Immunohistochemical polylysine-treated slides were placed in a 6-well plate, and 5×10 HepG2.B111 cells were added 5 Each well was allowed to slide cells for 72 hours. After the cells were fixed, two-step method was used for immunohistochemical staining. The primary antibody was rabbit anti-HBs / anti-H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com