Biological preparation method of intermediate of atazanavir
A technology for biological preparation and intermediates, applied in fermentation and other directions, can solve problems such as violation of basic principles, decreased enzyme activity and stability, and unfavorable energy conservation and environmental protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention provides an improved biological preparation method of atazanavir intermediates, which solves the danger and pollution problems in the production of atazanavir intermediates by chemical methods. Compared with chemical methods, its advantage is that it does not use flammable and Explosive chemical reagents do not use a large amount of organic solvents, which reduces pollution; the method of the invention also solves the problems of low substrate concentration, high energy consumption, and low efficiency in the production of atazanavir intermediates by traditional biological methods. Almac's technology has obvious advantages in substrate concentration. Compared with Codexis technology, the substrate concentration and enzyme dosage are at the same level, but by improving the coenzyme cycle system, the reaction temperature is lowered, the control risk is reduced, and a green and energy-saving reaction is realized.
[0024] The reaction flow chart of the inventi...
Embodiment 1
[0028] Embodiment 1 (enzyme reaction small test)
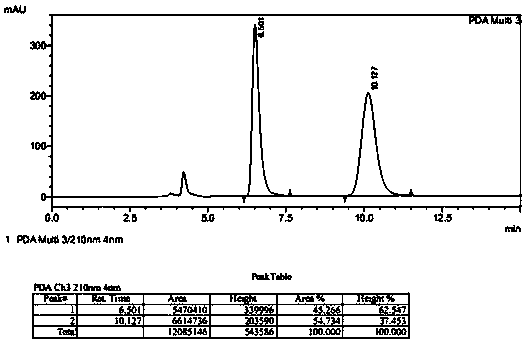
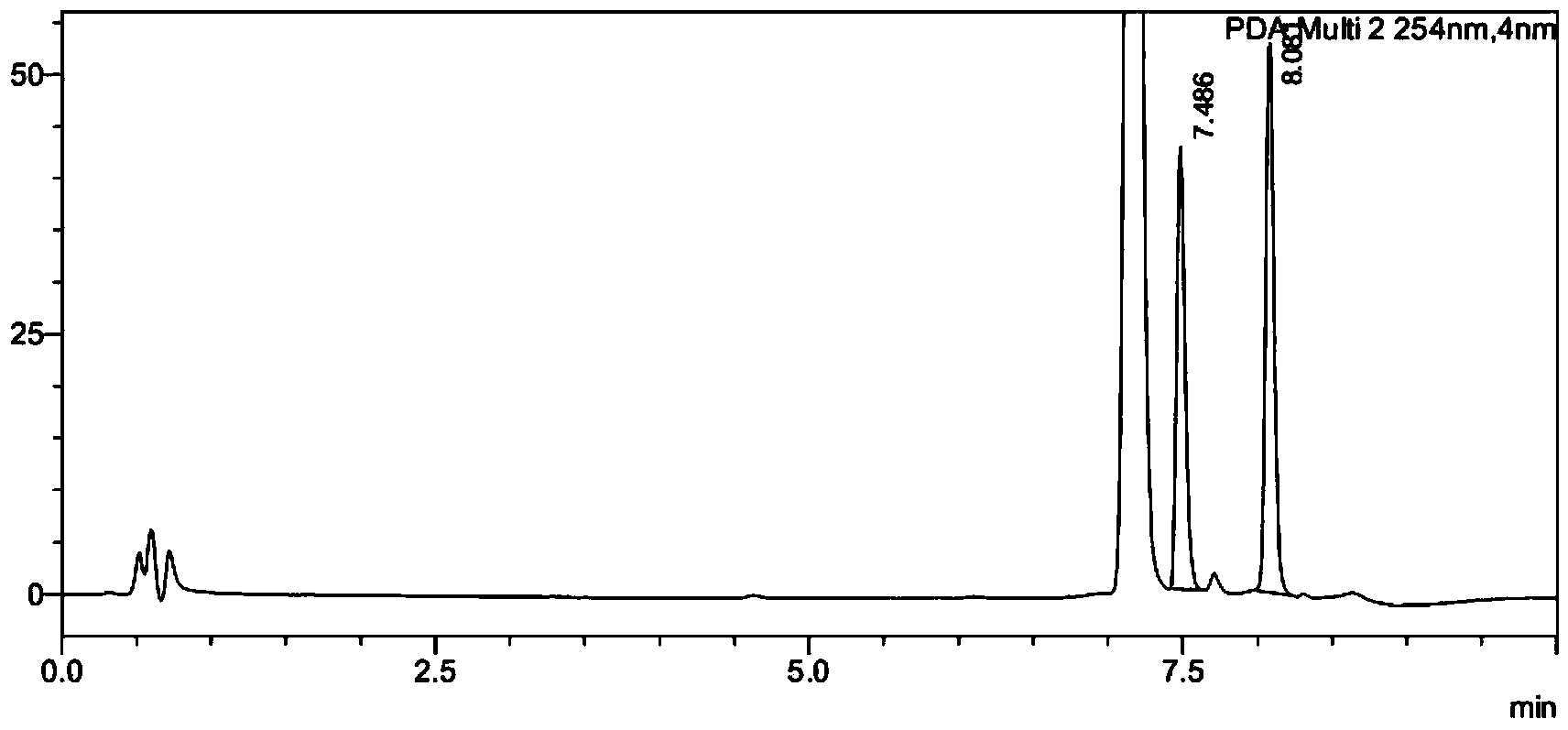
[0029] Add 100 mg of substrate ((S)-tert-butyl(4-chloro-3-carbonyl-1-phenylbutyl-2-yl)carbamate), 400 μL of co-solvent acetonitrile, 100 mg of glucose into a 5 mL reactor Stir well; weigh 3 mg of ketoreductase (KRED) enzyme powder, 3 mg of glucose dehydrogenase (GDH) and 0.36 mg of NAD in 1400 μL of pH 8.5 triethanolamine buffer solution, and add this mixed solution into the reactor Stir and start the reaction in a water bath at 33°C; take a sample for HPLC detection after 1 hour, and the conversion rate is 37%, and take a sample for HPLC detection after 20 hours, and the conversion rate is 96% (see figure 1 and figure 2 , the product corresponding to 7.48 minutes in the figure, and the substrate corresponding to 8.08 minutes).
Embodiment 2
[0030] Example 2 (enzyme reaction amplification)
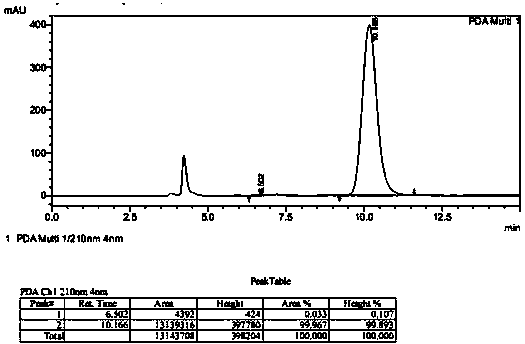
[0031] Add 100g substrate ((S)-tert-butyl (4-chloro-3-carbonyl-1-phenylbutyl-2-yl) carbamate), 170mL toluene and 100g glucose in 2L reactor, stir Uniform; Weigh 1.2g ketoreductase (KRED) enzyme powder, 1.2g glucose dehydrogenase (GDH) enzyme powder and 0.15g NAD and dissolve in 585mL pH8.5 triethanolamine buffer solution, and add this mixed solution to Stir in the reactor and start the reaction in a water bath at 33°C; after 24 hours, the conversion rate of the substrate is 97% as detected by HPLC. Add ethyl acetate and continue to stir and extract, combine the organic phases, dry and filter, and concentrate to obtain 85g of solid product, with a yield of 85%, a purity of 97.7%, and an optical purity of 99.8% (chiral HPLC) (chiral HPLC racemate standard product such as image 3 As shown, the product is as Figure 4 shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com