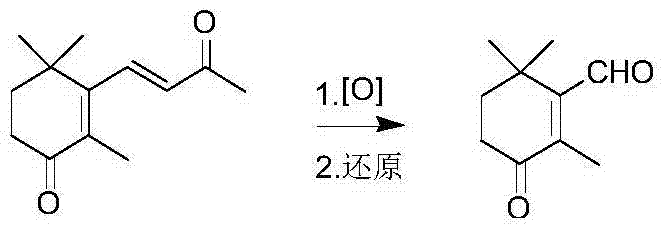
Preparation method of 2, 4, 4-trimethyl-3-formyl-2-cyclohexene-1-one
A formyl and cyclohexene technology, applied in the field of synthesis of organic intermediates, can solve the problems of difficult source of raw materials, large bromination pollution, etc., and achieve the effects of simple post-processing, fast reaction speed and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 61.8 grams of 4-oxo-β-ionone (0.3mol) and 200 milliliters of dichloromethane into a 500-mL four-neck flask, mechanically stir at -20°C, and adjust the oxygen flow to carry out the ozonation reaction. Gas chromatography Following the reaction, the raw materials disappeared in about 1.5 hours, and the ozonation was stopped at the end of the reaction. Keep the internal temperature around 0°C, add dropwise to 150 ml of aqueous solution containing 31.2 g of sodium bisulfite (0.3 mol), rise to 60°C after the addition and continue stirring for 1 hour. The sodium solution was adjusted to pH9, and the layers were allowed to stand. The aqueous phase was extracted with dichloromethane, the organic layers were combined, dried over anhydrous sodium sulfate, the dichloromethane was recovered by atmospheric distillation, and the 105-108°C / 1mmHg fraction was collected under reduced pressure to obtain 39.6 grams of light yellow liquid with a gas phase content of 98.4%. , yield 78.2...
Embodiment 2
[0029] Add 61.8 g of 4-oxo-β-ionone (0.3 mol) and 250 ml of methanol into a 500 mL four-neck flask, mechanically stir at -80 ° C, adjust the oxygen flow, and then carry out the ozonation reaction, and follow the reaction by gas chromatography , About 1 hour raw material disappears, and the ozonation is stopped after the reaction is finished. Keep the internal temperature at about 10°C, add the oxidizing solution dropwise to 55.8 grams of dimethyl sulfide (0.9 mol), raise it to room temperature and continue to stir for 6 hours, then first recover dimethyl sulfide, methanol, etc. Solvent, and then use a water pump to recover DMSO under reduced pressure, and finally collect the 105-108°C / 1mmHg fraction under reduced pressure to obtain 43.1 grams of light yellow liquid, the gas phase content is 97.2%, and the yield is 84.1%. Product nuclear magnetic spectrum is identical with embodiment 1.
Embodiment 3
[0031] Add 61.8 grams of 4-oxo-β-ionone (0.3mol) and 250 milliliters of ethanol into a 500-mL four-neck flask, mechanically stir at -40°C, adjust the oxygen flow to carry out the ozonation reaction, and follow the reaction by gas chromatography , About 1 hour raw material disappears, and the ozonation is stopped after the reaction is finished. Keep the internal temperature at about -10°C, add the oxidizing solution dropwise to 100 grams of triethyl phosphite (0.6mol), after the addition, raise it to room temperature and continue to stir for 5 hours. After the reaction, the water pump decompressed distillation to recover triethyl phosphite Esters, ethanol, etc., and then the oil pump decompressed to collect 105-108 ° C / 1mmHg fraction to obtain 43.7 grams of light yellow liquid, the gas phase content was 95.6%, and the yield was 83.9%. Product nuclear magnetic spectrum is identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com