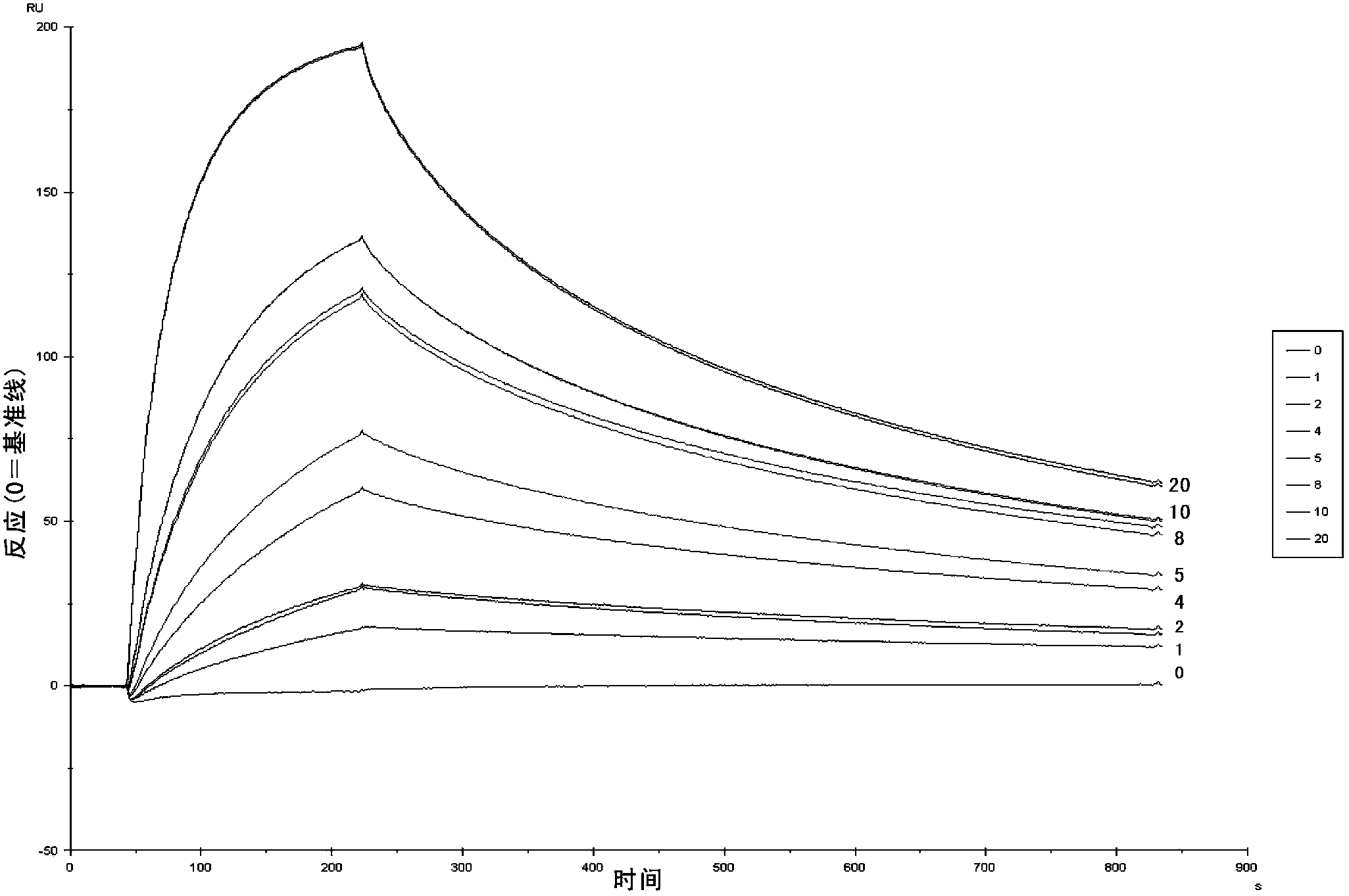
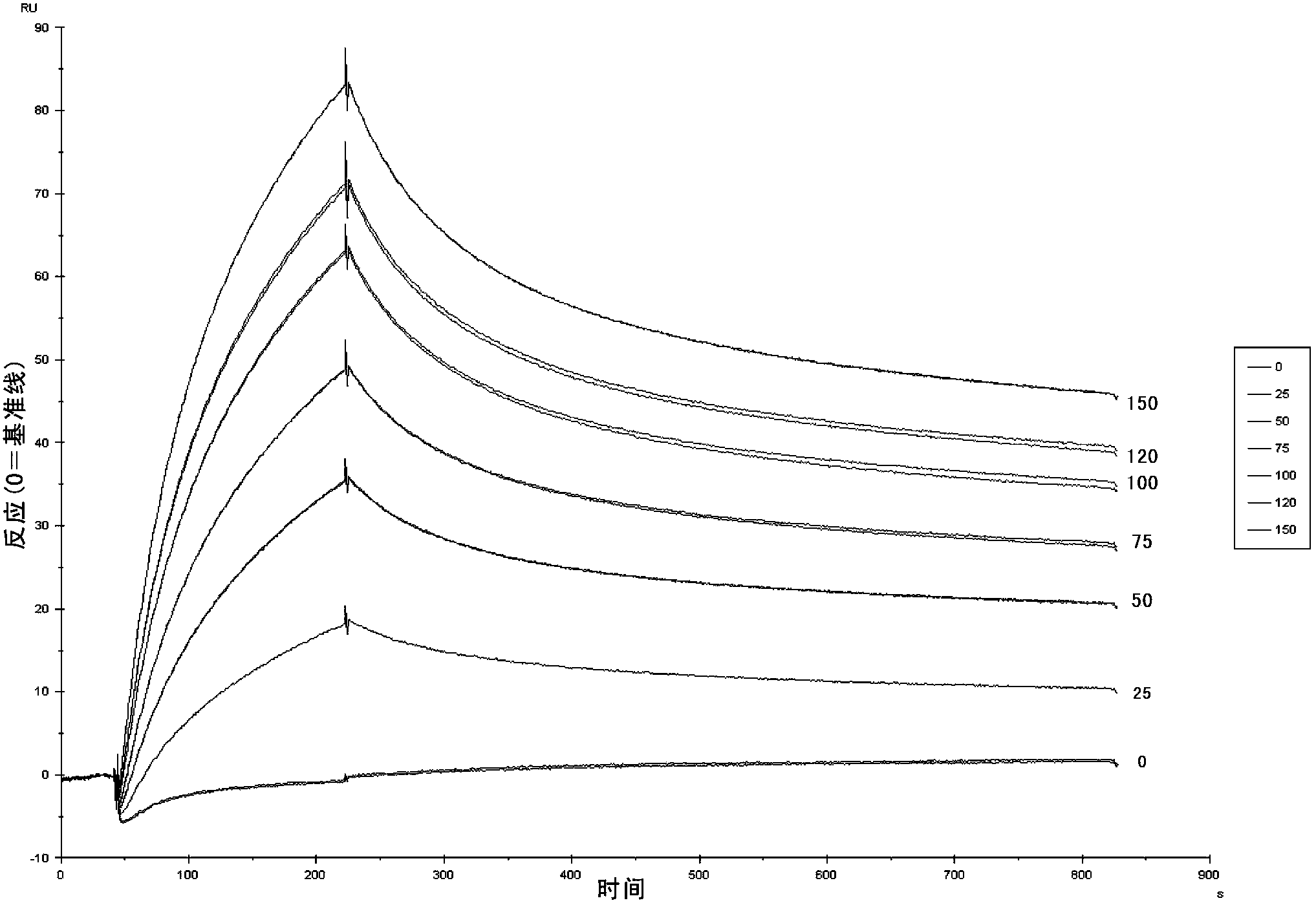
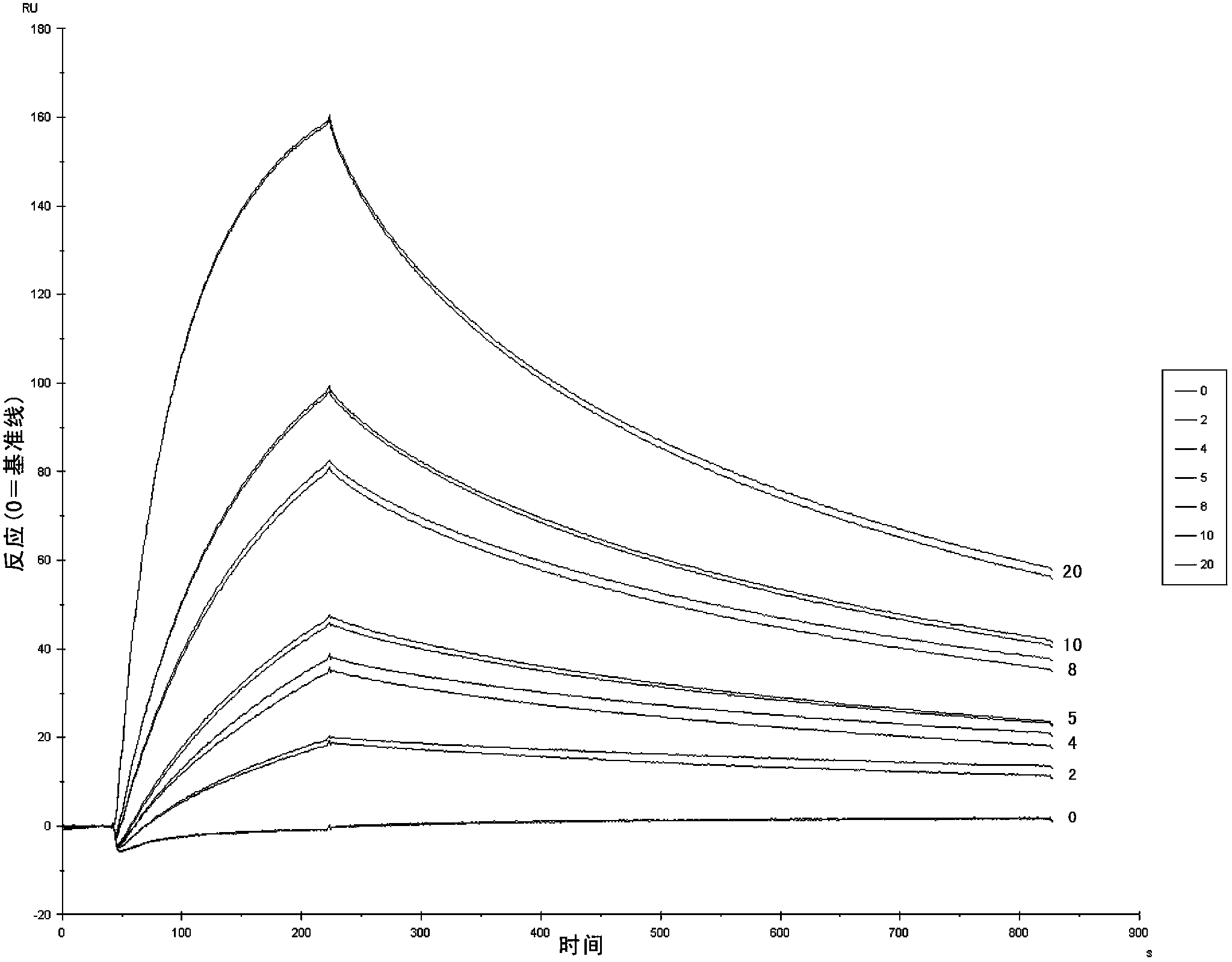
Nanometer antibodies to human cystatin C and application thereof
A technology of nanobodies and human cysts, applied in the fields of application, virus/bacteriophage, protease inhibitor immunoglobulin with anti-peptide structure, etc., can solve the problems of low sensitivity, complicated operation, poor reproducibility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0099] In an example of the present invention, the monoclonal antibody can be prepared by the following preparation method, which includes the steps of: (1) providing mice pretreated with adjuvant; (2) intraperitoneally inoculating the mouse with the said hybridoma cells and secrete monoclonal antibody; (3) extract ascites and separate and obtain said monoclonal antibody. As a preferred method, the method of isolating monoclonal antibody from ascites is as follows: collect ascites, precipitate with ammonium sulfate and octanoic acid, and then purify with Protein G prepacked chromatography column to obtain high-purity cystatin C monoclonal antibody .
[0100] In addition, the hybridoma cells can also be cultured and expanded in vitro according to conventional animal cell culture methods, so as to secrete the monoclonal antibody.
[0101] Reagent test kit
[0102] Those skilled in the art understand that an antigen may contain multiple epitopes (antigenic determinants), theref...
Embodiment 1
[0131] Embodiment 1, cystatin C recombinant expression
[0132] 1. Polymerase chain reaction (PCR) amplification of the full-length cystatin C gene
[0133] (1) Template: 293T total RNA of human kidney epithelial cells.
[0134] (2) Primer design: using the full-length mature cystatin C gene 363bp, design primers for amplifying the cystatin C gene after removing the signal peptide sequence, the upstream and downstream primers respectively carry endonucleases BamHI and XhoI sequence of restriction enzyme sites.
[0135] Upstream primer: 5' GGATCC AGTCCCGGCAAGCCG-3' (SEQ ID NO: 12);
[0136] Downstream primer: 5'-C CTCGAG CTAGGCGTCCTGACAGGT-3' (SEQ ID NO: 13);
[0137] (3) Total cellular RNA was extracted, and reverse-transcribed into cDNA using primer Oligo(dT)18 and reverse transcriptase.
[0138] The mature cystatin C gene (363bp) was obtained by RT-PCR, and connected to the cloning vector (pEASY-T1simple [purchased from Beijing Quanshijin Biotechnology Co., Ltd.]). A...
Embodiment 2
[0180] Embodiment 2, animal immunization
[0181] (1) Immunization of BALB / c mice
[0182] The mouse species is BALB / c. Mix 100 μg of recombinant cystatin C protein with an equal volume of Freund's complete adjuvant, emulsify completely, and inject subcutaneously in the back and feet of the mouse for immunization. Complete Freund's adjuvant was used for the first immunization, followed by incomplete Freund's adjuvant. One week after the fourth immunization, blood was collected from the orbit, the serum was separated, and the titer of anti-cystatin C protein antibody was measured. As detected by ELISA, the antibody titer of the mice after four immunizations was 1:512,000.
[0183] (2) ELISA steps
[0184] 1) Coating: Dilute the antigen (natural cystatin C) with 0.05M carbonate coating buffer (pH=9.6) to a protein content of 1.0 μg / ml. Add 0.1 ml to the reaction well of each polystyrene plate, overnight at 4°C, discard the solution in the well, and wash 3 times with 200 μl / wel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com