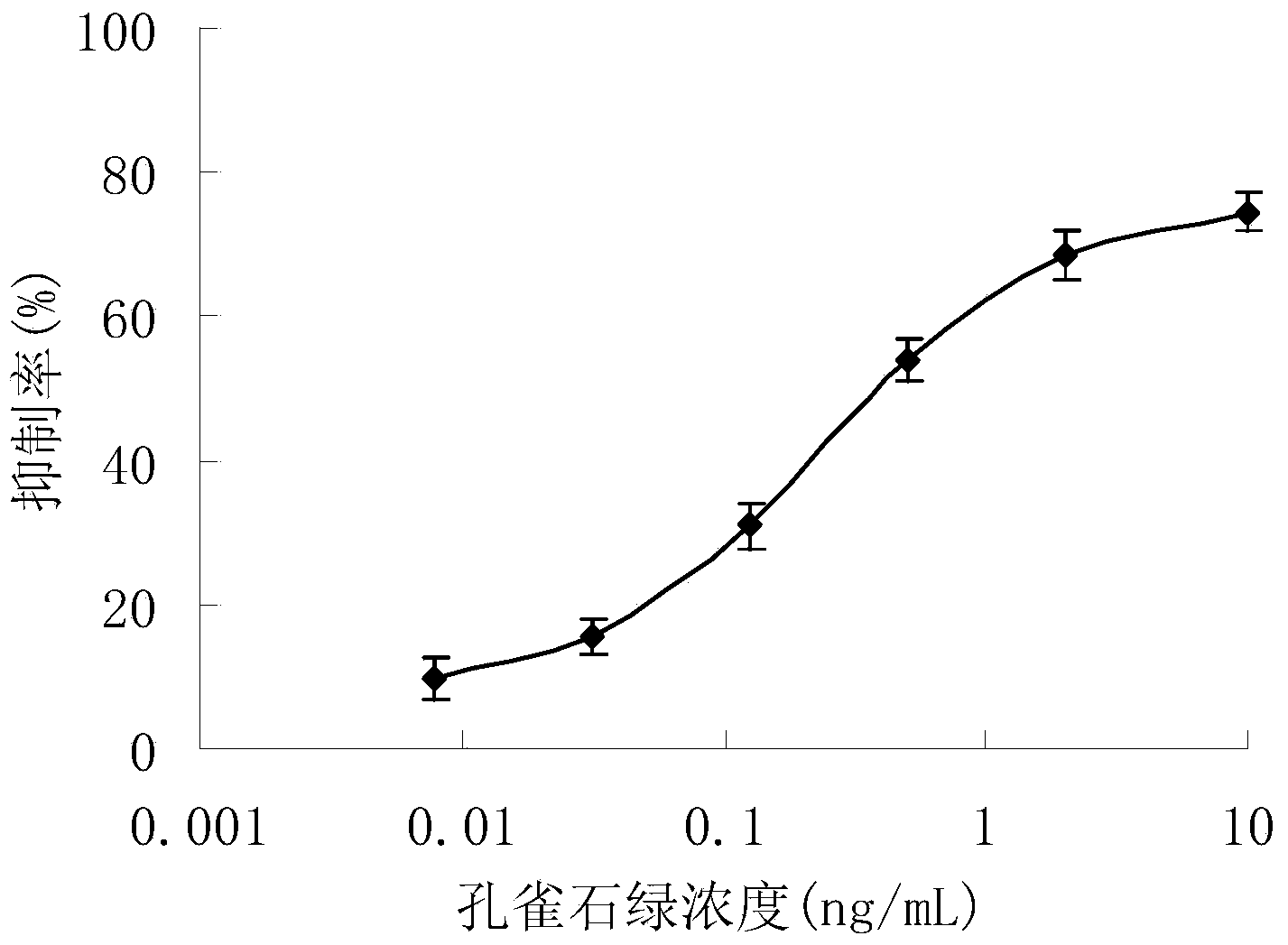
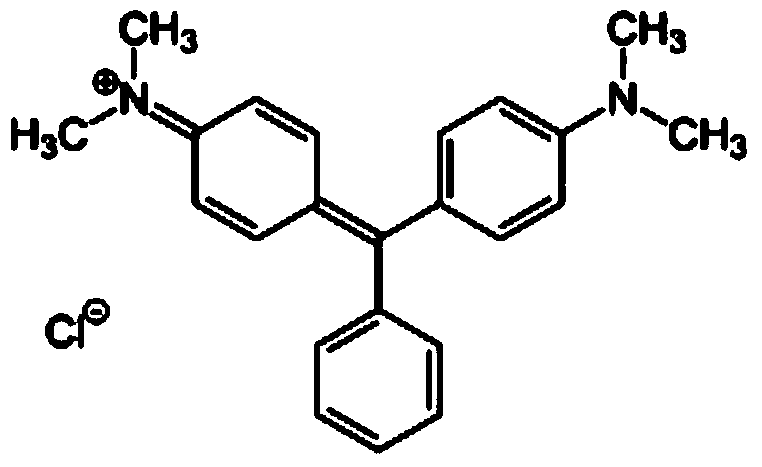
Malachite green artificial antigen and antibody, and preparation method and application thereof
A malachite green and hapten technology, which is applied in chemical instruments and methods, cyanide reaction preparation, organic compound preparation, etc., can solve the problems of cumbersome operation, inapplicable rapid detection, expensive equipment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
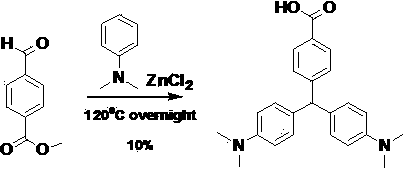
[0055] (a) 4-[bis(4-dimethylamino)phenyl]methylbenzoic acid
[0056]
[0057] Synthetic route of hapten (a):
[0058] Accurately weigh 1.8 g of methyl p-formylbenzoate and 3.7 g of zinc chloride into a 50 mL flask, then add 5.3 g of N,N-dimethylaniline, heat to 120 ° C, stir for 4 hours, and ethyl acetate Ester extraction and purification by silica gel column chromatography (ethyl acetate / n-hexane = 1:2) gave the target compound 4-[bis(4-dimethylamino)phenyl]methylbenzoic acid with a yield of 10%.
[0059] Example 2 Synthesis of malachite green hapten 5-{4-[bis(4-dimethylamino)phenyl]methyl}phenylvaleric acid
Embodiment 2
[0060] (b) 5-{4-[bis(4-dimethylamino)phenyl]methyl}phenylvaleric acid
[0061]
[0062] 1. Accurately weigh 10 g of p-hydroxybenzoic acid and dissolve in dichloromethane, add 7.8 ml of benzyl bromide and 9.3 ml of triethylamine at 0°C, stir at room temperature for 1-2 days, add saturated ammonium chloride at 0°C to quench The reaction was quenched, extracted with dichloromethane, concentrated, and purified on a silica gel column (ethyl acetate / n-hexane=1:3) to obtain the compound 4-hydroxymethylbenzoic acid benzyl ester;
[0063] 2. Dissolve 4.1g of the product in dichloromethane, add 7.9g of trichloroisocyanuric acid and 2.86g of NaHCO at 0°C 3 and 2,2,6,6-tetramethylpiperidine oxide, reacted for 5 minutes, purified by silica gel short column (ethyl acetate / n-hexane = 1:2), and the eluent was concentrated to obtain the compound 4-formylbenzoic acid Benzyl ester;
[0064] 3. Weigh 2.87g of wittig reagent and dissolve in tetrahydrofuran, slowly add 23.25ml of lithium hexam...
Embodiment 3
[0069] The hapten is coupled to ovalbumin (OVA) or hemocyanin (keyhole limpet hemocyanin, KLH) respectively by the activated ester method, the specific method is:
[0070] n=0, 4
[0071] Take 0.5 mmol of the above hapten, 0.625 mmol of N-hydroxysuccinimide (NHS) in a 25 ml round bottom flask and add 3 ml of anhydrous tetrahydrofuran, under N 2 Under protection, 4 ml of anhydrous tetrahydrofuran solution dissolved in 0.625 mmol N, N-dicyclohexylcarbodiimide DCC was added dropwise, stirred while dripping, stirred at room temperature for 4 h, purified by silica gel column to obtain hapten-activated ester .
[0072] The activated ester of the above hapten was dissolved in DMF to form solution A, and the carrier protein was dissolved in phosphate buffer solution to form solution B. Then solution A was slowly added to solution B under an ice bath. After stirring, react overnight at 4°C, and finally put the reaction solution into a dialysis bag, and dialyze in a PBS solution w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com