Preparation method and device of trimethyl borate-10
A technology of trimethyl borate and esterification reaction, applied in the field of trimethyl boron-10 acid, can solve the problems of large loss of trimethyl borate, difficulty in single treatment, complicated process, etc. Beautiful, good separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
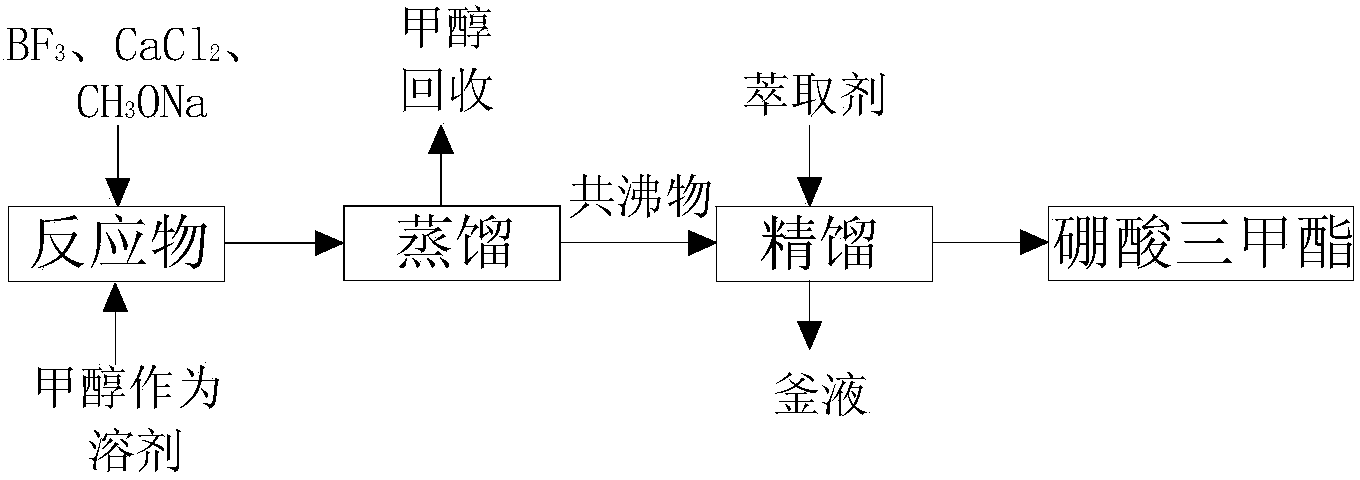
[0068] 1 Esterification reaction
[0069] (a) The reactor is purged with high-purity nitrogen for 5 to 10 minutes, and the reactants are mixed according to the molar ratio of the reactants 10 BF 3 :CaCl 2 :CH 3 Add ONa=1:1.5:3.0 to the reaction kettle, use methanol as solvent, so that the raw materials are evenly distributed in it, which is good for stirring, turn on the stirring device, and set the speed at about 150r / min;
[0070] (b) The reaction temperature is set to 70°C, and the reaction time is set to 30h;
[0071] 2 azeotropic distillation
[0072] (a) carry out azeotropic rectification to reaction product, temperature 70 ℃;
[0073] (b) Collect fractions at 50-60°C, and then continue to heat up to recover methanol for recycling.
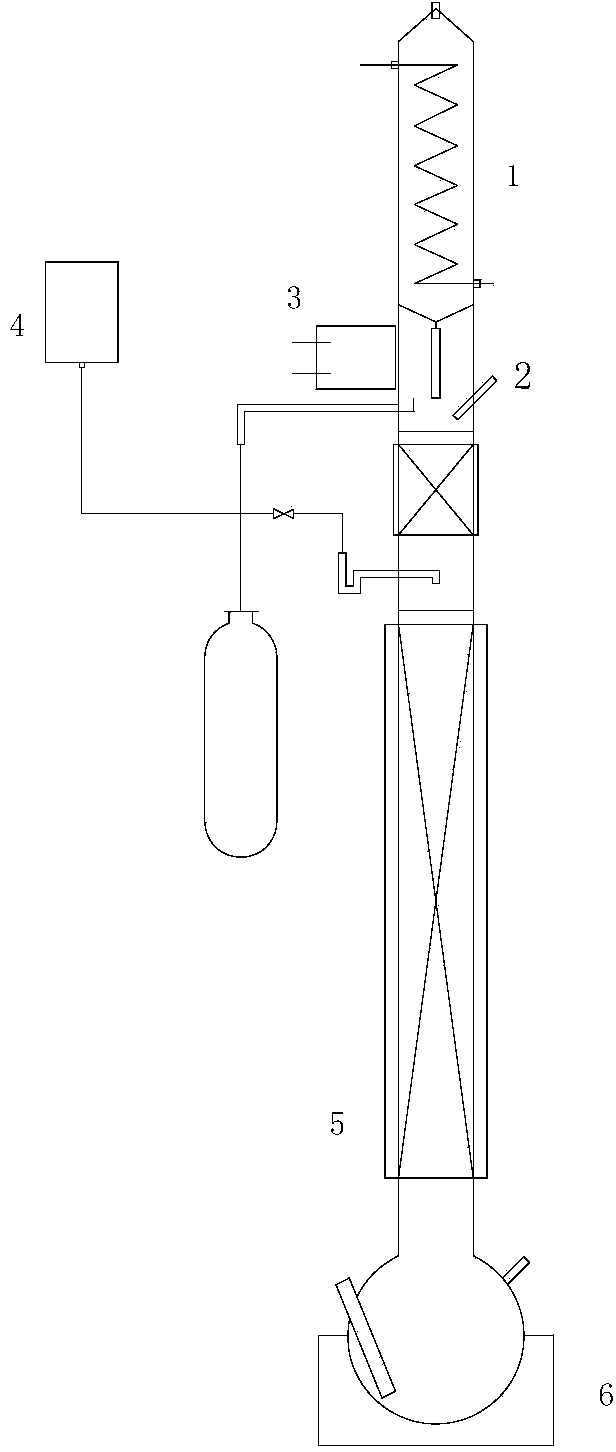
[0074] 3 extractive distillation
[0075] (a) Add the distillate to the kettle of the extractive distillation column, turn on the power supply of 6 heating packs and 1 condensate water switch, in order to ensure the heat transfer perf...
Embodiment 2
[0080] 1 Esterification reaction
[0081] (a) The reactor is purged with high-purity nitrogen for about 5 to 10 minutes, and the reactants are mixed according to the molar ratio of the reactants 10 BF 3 :CaCl 2 :CH 3 Add ONa=1:1.7:3.4 to the reaction kettle, use methanol as the solvent, so that the raw materials are evenly distributed in it, which is good for stirring, turn on the stirring device, and set the speed at about 200r / min;
[0082] (b) Set the reaction temperature to 60° C. and the reaction time to 35 h.
[0083] 2 azeotropic distillation
[0084] (a) The reaction product is subjected to azeotropic distillation, and the temperature is set to 80° C.;
[0085] (b) Collect fractions at 50-60°C, and then continue to heat up to recover methanol for recycling.
[0086] 3 extractive distillation
[0087] (a) Add the distillate to the kettle of the extractive distillation column, turn on the power supply of 6 heating packs and 1 condensate water switch, in order to e...
Embodiment 3
[0092] 1 Esterification reaction
[0093] (a) The reactor is purged with high-purity nitrogen for about 5 to 10 minutes, and the reactants are mixed according to the molar ratio of the reactants 10 BF 3:CaCl 2 :CH 3 Add ONa=1:1.8:3.6 to the reaction kettle, use methanol as the solvent, so that the raw materials are evenly distributed in it, which is convenient for stirring, turn on the stirring device, and set the speed at about 300r / min;
[0094] (b) The reaction temperature is set to 50°C, and the reaction time is set to 45h;
[0095] 2 azeotropic distillation
[0096] (a) carry out azeotropic rectification with reaction product, temperature 90 ℃,
[0097] (b) Collect fractions at 50-60°C, and then continue to heat up to recover methanol for recycling.
[0098] 3 extractive distillation
[0099] (a) Add the distillate to the kettle of the extractive distillation column, turn on the power supply of 6 heating packs and 1 condensate water switch, in order to ensure the h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com