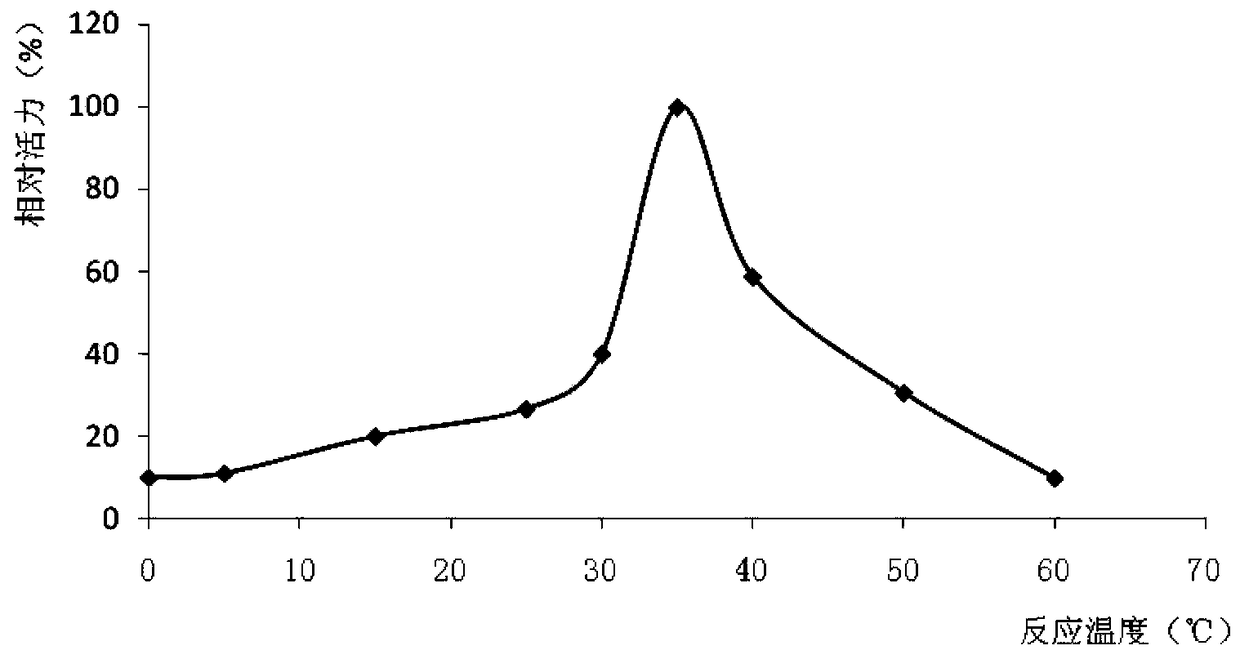
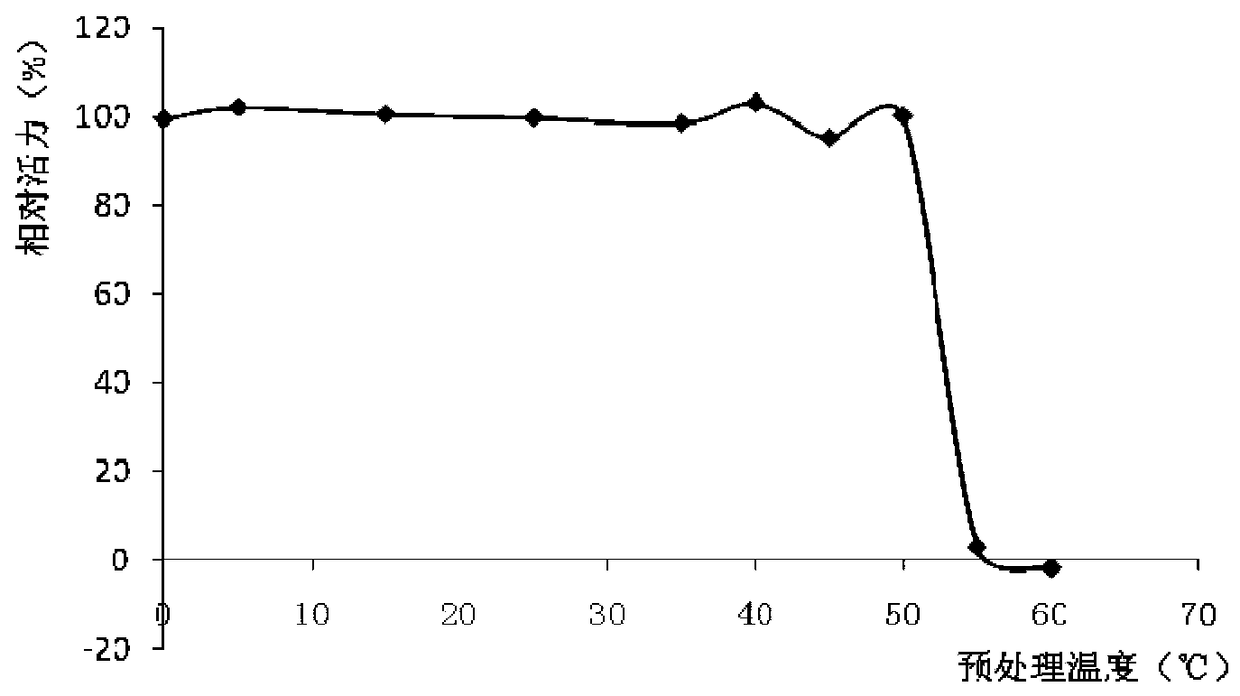
A low-temperature organic solvent-resistant lipase derived from Bacillus mojave
An organic solvent and lipase technology, applied in the biological field, can solve the problem of no Bacillus lipase, etc., and achieve the effects of broad organic solvent tolerance, good heat resistance, and broad pH stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Cloning of lipase gene
[0056] 1. Acquisition of Conserved Sequences
[0057] According to the conservative sequences P(V / I)V(M / L)(V / L)HG and AHS(M / Q)GG of the conserved region Oxyanion hole and Activity center of the lipase gene, the designed degenerate primers are shown in Table 1:
[0058] Table 1 Primer Information
[0059]
[0060]
[0061] The genome of Mojave bacillus was extracted by SDS method (Fang Weiguo, Acta Applied and Environmental Biology, 2002, 8(3): 305-307). Upstream primers and downstream primers were randomly combined, and there were 12 primer pairs in total. Using the genome as a template, the following degenerate PCR reactions were performed using the above primer pairs, respectively. PCR system: rTaq 0.5uL, 10×PCR buffer 5uL, dNTP mixture 8uL, primer 11uL, primer 21uL, genome 0.3uL, water 34uL. Reaction conditions: 94°C for 1s, 94°C for 1min, 37°C for 1s, 37°C for 30s, 72°C for 2min20s, 72°C for 1min, 35 cycles (95°C for 1min...
Embodiment 2
[0076] Embodiment 2: Construction of lip2-2 recombinant Escherichia coli
[0077] 1. Cloning of lip2-2 expression gene
[0078] Use the Bacillus mojave genome as a template and use the cloning primers lip-U / lip-D in the table to perform PCR. See Example 1 for the PCR reaction system. Reaction conditions: 94°C for 5min, 35 cycles (95°C for 30s, 47°C 30s, 1min at 72°C), 10min at 72°C, keep warm at 4°C.
[0079] 2. Digestion of genes and vectors and purification of digested products
[0080] Recover the above PCR product from the gel, and simultaneously digest the PCR product and plasmid pET24a with EcoRI and Hind III, enzyme digestion system: 20.0uL gel recovery product (or vector), 21.0uL ddH 2 O, 5.0uL 10×M Buffer, 2.0uL EcoRI and 2.0uL HindⅢ, digestion at 37°C overnight. (Make 2 tubes of 50uL enzyme digestion system for the recovered product from gel injection, and 2 tubes for the carrier), and purify the enzyme digestion product with Omega PCR Product Purification Kit. ...
Embodiment 3
[0087] Example 3: Induced expression of recombinant Escherichia coli and separation and purification of target protein
[0088] 1. Induced expression of recombinant bacteria
[0089] Inoculate a ring of BL21 (pET24a-lip2-2) into LB liquid medium containing kanamycin and culture it for about 8 hours, then inoculate BL21 (pET24a-lip) in 50 mL of LB liquid medium ( containing 50μg / mL kanamycin), 37°C, 200rpm shaking culture, while using the empty host BL21 (DE3) as a control. Waiting for OD 600 Increase to about 0.4-0.6, add a final concentration of 1.0mM IPTG, culture at 37°C, 200rpm for 5h, and induce the expression of foreign proteins.
[0090] 2. Method for Determination of Lipase Activity
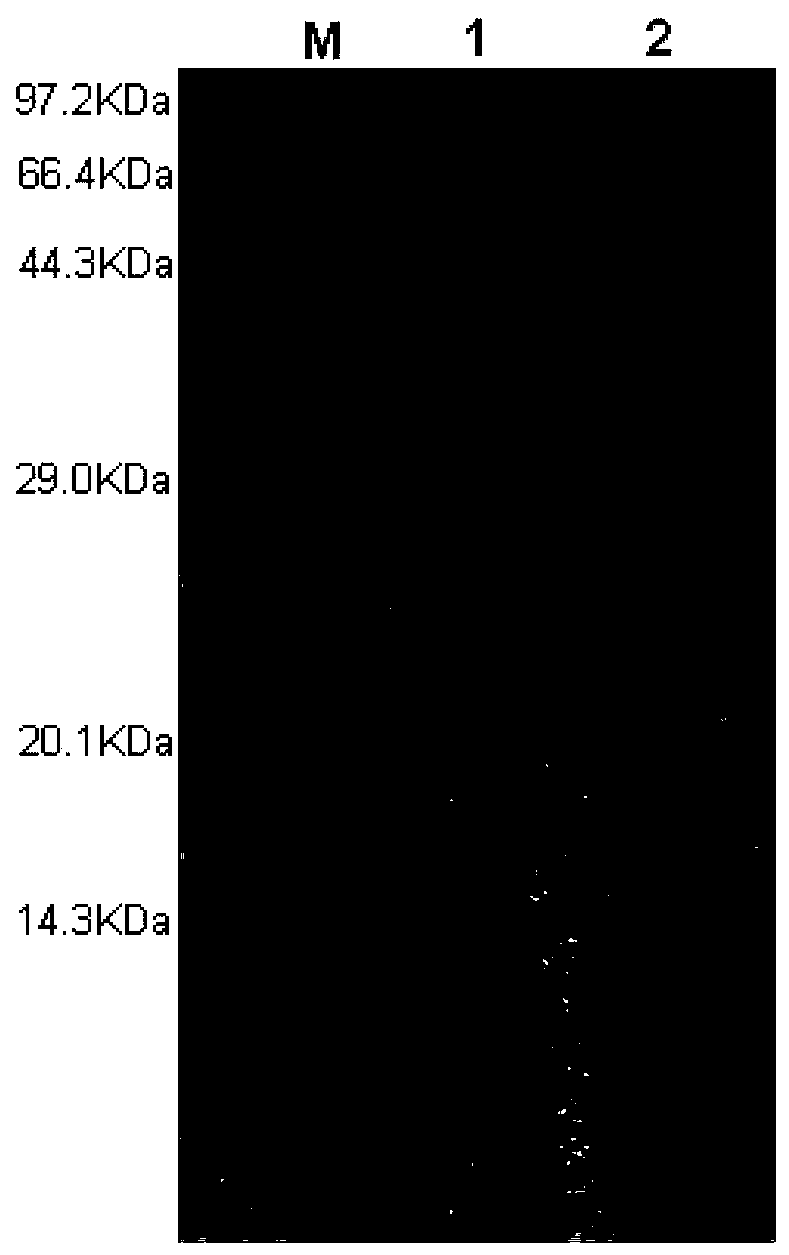
[0091] Low-temperature lipase activity was determined by colorimetry. With p-nitrophenyl palmitate (p-NPP) as the substrate, the enzymatic activity is calculated by the amount of p-nitrophenol (p-NP) produced by enzymatic hydrolysis per unit volume of enzyme solution per unit time. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com