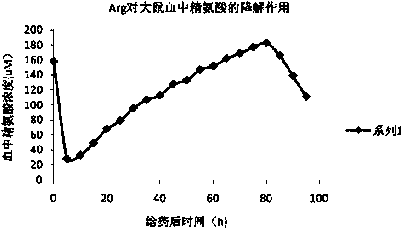
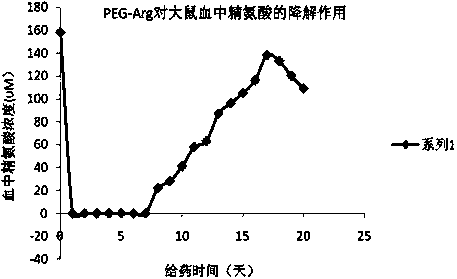
Sulfhydryl modified recombinant human arginase I, as well as preparation method and application thereof
A technology of arginase and sulfhydryl modification, applied in the field of chemical modification of recombinant proteins, can solve the problem of immunogenicity, liver function decompensation and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Preparation of recombinant human arginase I
[0041] Human type Ⅰ arginase gene (hAI) was amplified by PCR, inserted into the expression vector pET30a of Escherichia coli, the recombinant expression plasmid pET30a-hAI was constructed, transformed into the host strain of Escherichia coli BL21 (DE3), and the genetically engineered strain of Escherichia coli BL21 (DE3 ) (pET30a-hAI), expressed after IPTG induction. SDS-PAGE analysis showed that the induced BL21(DE3)(pET30a-hAI) had obvious protein expression at about 35kD. After fermentation and purification, the final purity is over 95%. Refer to the literature for detailed steps (Masaki IKEMOTO, Masayoshi TABATA, Toshio MIYAKE, et al. Expression of human liver arginase in Escherichia coli. Biochem, J.1990, (270): 697-703)
Embodiment 2
[0042] Example 2. Preparation of PEG (40KD)-Arg whose modification site is at the 303rd cysteine sulfhydryl group of Arg
[0043] Adjust the protein concentration of Arg to 4.0mg / ml, adjust the pH value of the protein solution to 7.5, add Y-type PEG-MAL (40KD) equivalent to 2 times the molar number of Arg, and stir at 4°C for 12 hours. The modification efficiency in the reaction was detected by SDS-PAGE method. After 12 hours of reaction, the SDS-PAGE results showed that more than 80% of Arg was modified, and the single modified component accounted for 50-70% of the modified components. The time was further prolonged, and the modification rate No longer improve. After the reaction was terminated, the precipitate was discarded by centrifugation, and the supernatant was directly applied to the Sephacryl-S300 column, and 50mM glycine-sodium hydroxide (pH9.8) buffer solution plus 0.15M sodium chloride was used as the eluent. After this step, the unmodified Arg molecule removal....
Embodiment 3
[0044] Embodiment 3. Preparation of PEG-Arg injection
[0045] After the PEG-Arg stock solution obtained in Example 2 was used to determine the protein concentration by the Lowry method, 5000 mg PEG-Arg was added to the liquid containing the following substances: 6.78 g of disodium hydrogen phosphate, 0.17 g of sodium dihydrogen phosphate, 0.1 ml of Tween 80, Sodium chloride 5.844g. After sterile filtration, it is divided into 2ml vials, and the filling volume of each bottle is 1ml.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com