Salicylamide derivatives and preparation method thereof
A technology of salicylamino and alkanoyl salicylamino is applied in the field of salicylic acid amide derivatives and pharmaceutically acceptable salts thereof, and can solve the problems of increased subsequent processes, complicated processing processes, poor product quality and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
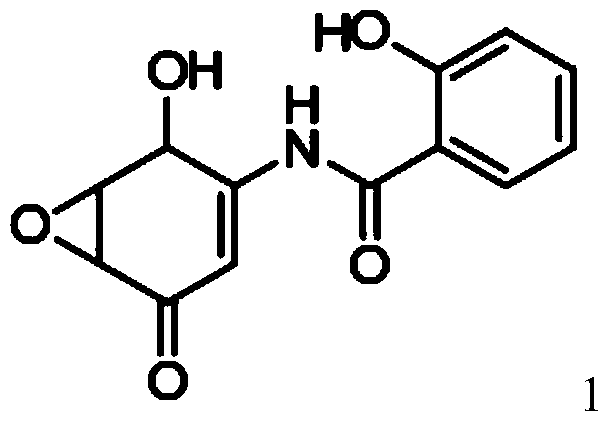
[0137] Example 1: Preparation of 5,6-epoxy-4-hydroxyl-3-salicyloylamino-2-cyclohexenone (DHM2EQ)
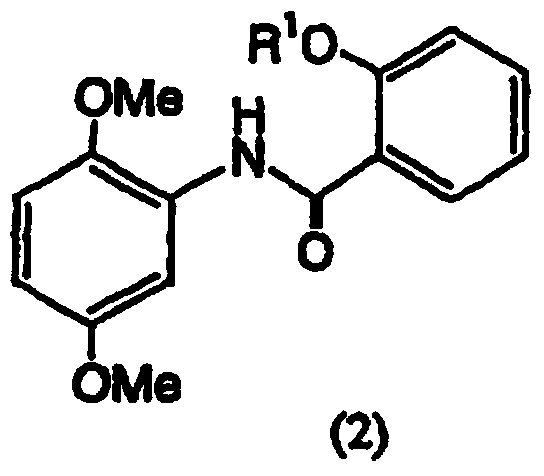
[0138] Step 1: Synthesis of N-(2-acetoxybenzoyl)-2,5-dimethoxyaniline
[0139]
[0140] 2,5-Dimethoxyaniline (10.0 g, 65.3 mmol) was dissolved in pyridine (100 ml). Under ice-cooling, a solution of O-acetylsalicyloyl chloride (13.0 g, 65.3 mmol) in ethyl acetate (50 ml) was added thereto over 15 minutes, followed by stirring at the same temperature for 15 minutes. After adding water (10ml) to the reaction solution to stop the reaction, add ethyl acetate (500ml), then successively add 3 equivalents of hydrochloric acid (500ml), water (500ml), 2% aqueous sodium bicarbonate (500ml) and water (500ml )washing. The ethyl acetate layer was dried over Glauber's salt, concentrated under reduced pressure and dried in vacuo to obtain the title compound (19.8 g) as a light yellow syrup. The compound was used directly in the following step without further purification. After separat...
Embodiment 2
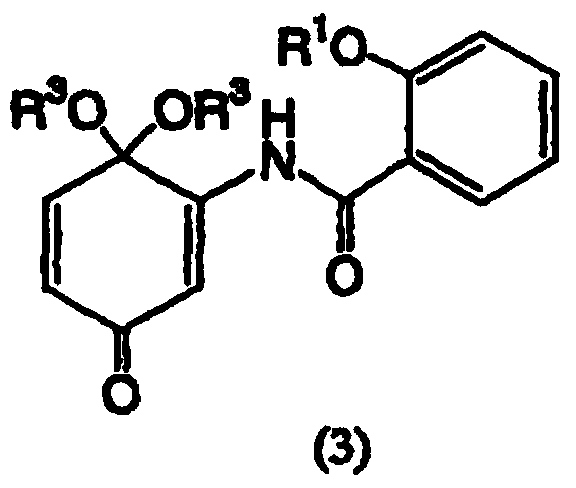
[0158] Example 2: Synthesis of 5,6-epoxy-4,4-dimethoxy-3-salicyylamino-2-cyclohexenone
[0159] 3-(O-acetylsalicyloylamino)-4,4-dimethoxy-2,5-cyclohexadienone (10.9 g, 33.0 mmol) was dissolved in dimethylformamide (200 ml) ; Under ice-cooling, add 396mmol hydrogen peroxide (add with 30% hydrogen peroxide aqueous solution form) and the sodium carbonate (165ml) of 1mol / L wherein, stir reaction 2 hours at same temperature;
[0160] Add ethyl acetate (500ml) to the reaction solution, wash with 1 equivalent of hydrochloric acid (300ml), 10% aqueous sodium thiosulfate solution (300ml×2), 10% saline (300ml) successively, and wash the ethyl acetate layer with Glauber's salt is dried, then vacuum-dried to obtain a light yellow solid powder;
Embodiment 3
[0163] Example 3: Synthesis of 5,6-epoxy-4,4-dimethoxy-3-salicyylamino-2-cyclohexenone
[0164] 3-(O-acetylsalicyloylamino)-4,4-dimethoxy-2,5-cyclohexadienone (10.9 g, 33.0 mmol) was dissolved in dimethylformamide (200 ml) ; Under ice-cooling, add 330mmol hydrogen peroxide (add with 30% hydrogen peroxide aqueous solution form) and the sodium carbonate (165ml) of 1mol / L wherein, stir reaction 2 hours at same temperature;
[0165]Add ethyl acetate (500ml) to the reaction solution, wash with 1 equivalent of hydrochloric acid (300ml), 10% aqueous sodium thiosulfate solution (300ml×2), 10% saline (300ml) successively, and wash the ethyl acetate layer with Glauber's salt is dried, then vacuum-dried to obtain a light yellow solid powder;
[0166] The light yellow solid powder obtained by drying was dissolved in a mixed solvent of acetone-petroleum ether (the volume ratio of the two was 8:1), and an equal volume of saturated saline was added to extract twice, the water layer was di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com