Antioxidant peptide sourced from limnonectes fragilis as well as gene and application thereof
A technology of crispy big-headed frogs and antioxidant peptides, applied in the field of biomedicine, to achieve good application prospects, low hemolytic activity, and small molecular weight effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Gene cloning of three kinds of crispy big-headed frog antioxidant peptides Fragilin-A1, Fragilin-B2, Odorranain-Q-Lf:
[0051] 1 Extraction of total RNA from skin of crispy frog skin:
[0052] ① Take 300 mg of skin tissue of crispy-skinned frog, put it into a mortar and add liquid nitrogen to grind it into powder, transfer it to an EP tube, add 1 ml of total RNA extraction buffer (Trizol, product of Invitrogen, USA), mix well, Then centrifuge at 12000 rpm for 10 min at 4°C.
[0053] ② Centrifuge to get the supernatant, add 0.2 ml chloroform solution, mix vigorously, let stand at room temperature for 10 minutes, then centrifuge at 4°C, 12000 rpm for 10 minutes, discard the precipitate.
[0054] ③ Add an equal volume of isopropanol to the supernatant, place at room temperature for 10 minutes, centrifuge at 12,000 rpm at 4°C for 10 minutes, collect the precipitate, wash it once with 75% (V / V) ethanol, and dry it. The precipitate at the bottom of the tube is brittle. Tota...
Embodiment 2
[0106] Chemical synthesis of three antioxidant peptides Fragilin-A1, Fragilin-B2, and Odorranain-Q-Lf from the crispy frog:
[0107] 1. The chemical synthesis method of three kinds of crispy big-headed frog antioxidant peptides Fragilin-A1, Fragilin-B2, and Odorranain-Q-Lf: according to the amino acid sequence of the mature peptide derived from the gene, synthesize it with an automatic peptide synthesizer (433A, Applied Biosystems) The whole sequence was desalted by HPLC reverse phase column chromatography.
[0108] 2. The molecular weight was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF).
[0109] 3. The purity of the three purified antioxidant peptides Fragilin-A1, Fragilin-B2, and Odorranain-Q-Lf of crispy-headed frogs was identified by high-performance liquid chromatography (HPLC), and the molecular weight was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI -TOF), th...
Embodiment 3
[0114] Pharmacological experiments of three antioxidant peptides Fragilin-A1, Fragilin-B2, Odorranain-Q-Lf of crispy big-headed frog:
[0115] 1. Fragilin-A1, Fragilin-B2, Odorranain-Q-Lf antioxidant activity assay:
[0116] 1.1 DPPH radical scavenging activity (DPPH radical scavenging assay)
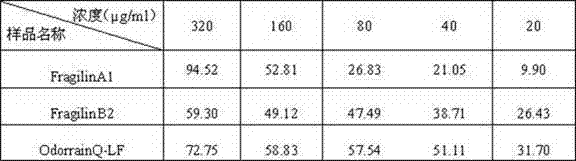
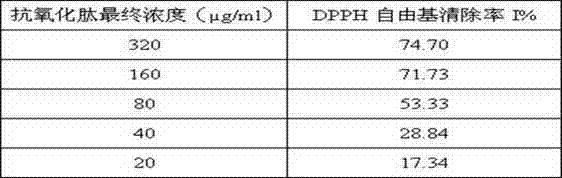
[0117] Weigh a certain amount of DPPH (2,2-diphenyl-1-picrylhydrazyl hydrate, Sigma, USA), dissolve it in methanol, and make 6×10 -5 The solution of M is prepared and used immediately. Mix 48 μl DPPH solution with 2 μl sample (2 mg / ml) (the mass ratio of final sample to DPPH is 3:1), let stand in the dark at room temperature for 30 min, and measure the absorbance at 517 nm. In the blank control group, the sample to be tested was replaced by the sample dissolution medium. The experiment was done in triplicate, and methanol was used when the UV spectrophotometer was zeroed.
[0118] DPPH·clearance rate (%) = (AB-AA ) / A B×100 (AB: absorbance value of blank control group; AA: absorbanc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com