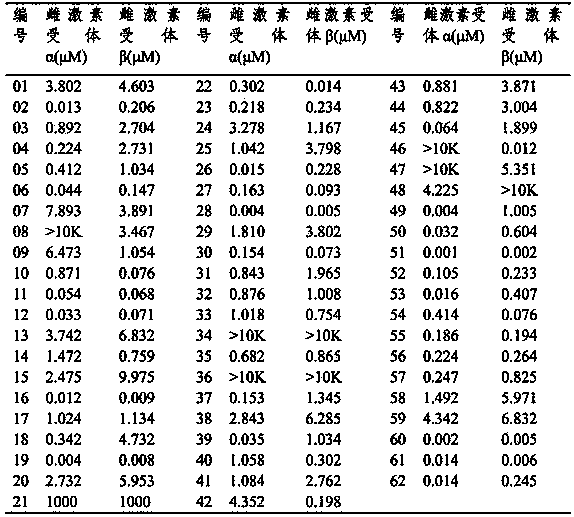
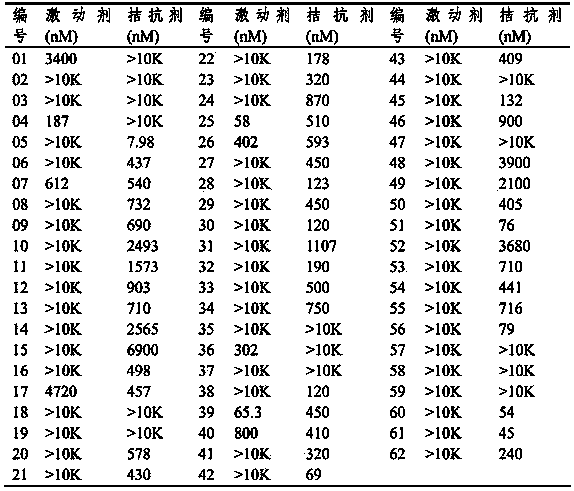
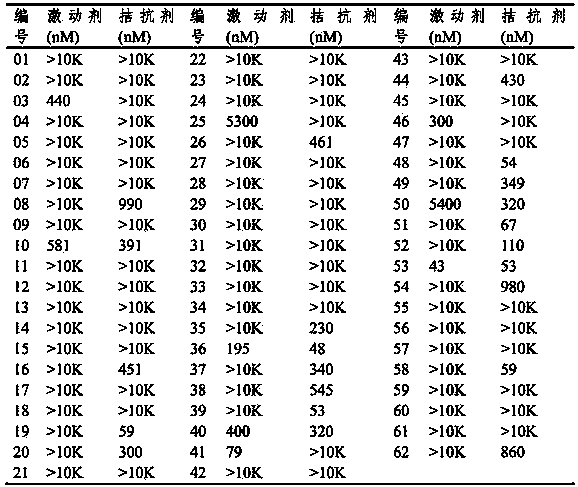
Thienothio compound and application thereof
A compound, thiophene technology, applied in the field of medicine, can solve problems such as reduced bioavailability and prolongation of cardiac QT interval
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: 1-(7-bromo-1,4-dihydrothieno[3',2':5,6]thiapyro[4,3-c]pyrazole-3-carbonyl)-4- Preparation of (4-methylphenyl)piperazine (compound number 01)
[0026] Step A: Preparation of 5,6-dihydro-4H-thieno[2,3-b]thiopyran-4-one
[0027] Add 4.64g (0.04mol) 2-mercaptothiophene and 80mL tetrahydrofuran into a 100mL round bottom flask, add 11mL triethylamine dropwise with stirring, then add 3.3mL acrylic acid, and heat to reflux for 12h. After the reaction was completed, tetrahydrofuran was distilled off. After cooling, 40 mL of ethyl acetate and 20 mL of water were added, the pH was adjusted to 2 with 18% hydrochloric acid, and the organic layer was collected. The aqueous layer was extracted with ethyl acetate, and the organic layers were combined and dried over anhydrous magnesium sulfate. After suction filtration, the filtrate was evaporated to remove the solvent, and a brown solid was precipitated after cooling. Petroleum ether was recrystallized to obtain a white ...
Embodiment 2
[0046] Example 2: 1-(7-bromo-1,4-dihydrothieno[3',2':5,6]thiapyrano[4,3-c]pyrazole-3-carbonyl)-4- Preparation of (diphenylmethyl)piperazine (compound number 02)
[0047] According to the method of Example 1, 1-(7-bromo-1,4-dihydrothieno[3',2':5,6]thiopyrano[4,3-c]pyrazole-3-carbonyl was obtained )-4-(diphenylmethyl)piperazine 0.77g, yield 35%. m.p.: 175-176℃; IR(KBr,cm -1 ):3420,2921,2851,1607,1451,1384,1262,1154,1027,997,877,831,705; 1 H-NMR (600MHz, CDCl 3 ):δ2.44(4H,m),3.79(4H,m),4.16(2H,s),4.25(1H,s),7.19(2H,d,J=8.4Hz),7.28(4H,dd, J=7.2Hz,8.4Hz),7.41(4H,d,J=7.2Hz); ESI-MS(m / z):551.2[M+H] + .
Embodiment 3
[0048] Example 3: N,N-diethyl-7-bromo-1,4-dihydrothieno[3',2':5,6]thiapyrano[4,3-c]pyrazole-3- Preparation of amides (compound number 03)
[0049] According to the method of Example 1, N,N-diethyl-7-bromo-1,4-dihydrothieno[3',2':5,6]thiopyrano[4,3-c]pyridine was prepared Azole-3-amide (Compound No. 03) 0.75 g, yield 50%. m.p.:147-148℃;IR(KBr,cm -1 ):3212,2958,2917,2849,1735,1583,1535,1508,1485,1461,1375,1215,1159,967,849; 1 H-NMR (600MHz, CDCl 3 ):δ1.25(6H,m),3.54(4H,m),4.12(2H,s),7.72(1H,s); ESI-MS(m / z):372.0,374.0[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com