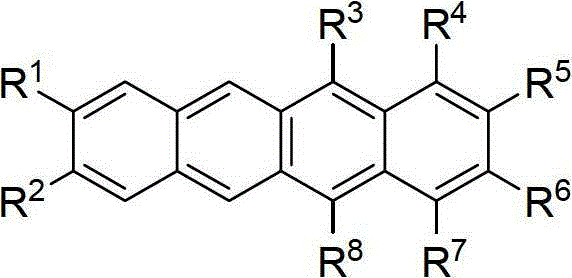
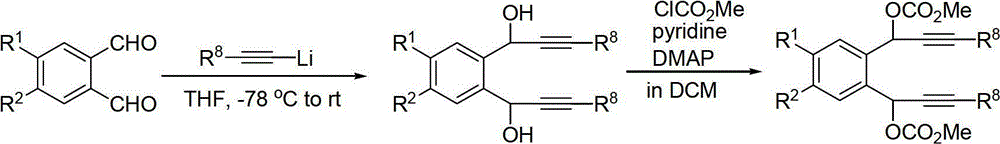
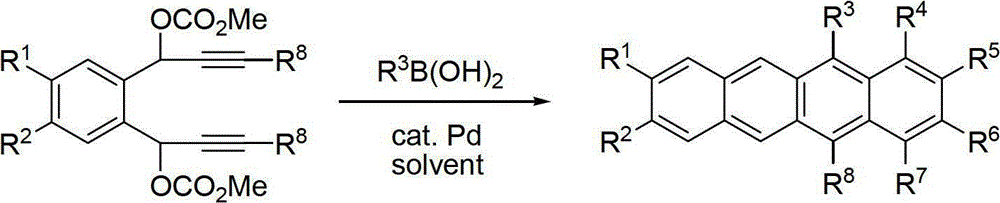
Synthetic method of tetracene and pentacene compounds
A synthetic method and compound technology, which is applied in the field of synthesizing aryl-substituted or alkyl-substituted naphthacene and pentacene compounds, can solve the problems of large substrate limitations, low yield, and many steps, and achieve operational Convenience, high yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of 5,12-diaryltetracene (5,12-diaryltetracene) from o-phthalaldehyde, phenylacetylene, and arylboronic acid
[0026] 1) Synthesis of raw material bispropargyl carbonate 1a
[0027]
[0028] Under the protection of argon, add phenylacetylene (120mmol, 13.2mL) and tetrahydrofuran (120mL) sequentially to a dry 250mL three-neck flask with a stirring bar, then cool to -78°C in a dry ice / acetone bath, and slowly add 2.5M n-Butyllithium / n-hexane solution (110mmol, 44mL), the dropwise addition was completed, and after stirring for 30 minutes, o-phthalaldehyde (50mmol, 6.71g) was added, reacted for 2 hours, and TLC detected that the reaction was completed; saturated NH 4 Quenched with Cl solution, extracted with ethyl acetate, anhydrous Na 2 SO 4 Dry, filter, spin dry, and separate by silica gel column chromatography, eluent: petroleum ether: acetone = 5:1, to obtain 17.03 g of yellow viscous liquid, yield 100%.
[0029]Add the diol (5mmol, 1.7g), dich...
Embodiment 2
[0033] Example 2: Synthesis of 5-phenyl-12-(p-chlorophenyl)naphthacene (5-(4-Chlorophenyl)-12-phenyltetracene)
[0034]
[0035] Under argon protection, bispropargyl carbonate 1a (0.2mmol, 91mg), p-chlorophenylboronic acid (0.8mmol, 125.1mg) and tetrahydrofuran (5mL) were successively added into a 25mL Schlenk reaction tube with a stirring bar to dissolve, and added Tetrakistriphenylphosphine palladium (0.01mmol, 12mg), react at 100°C for 3h, TLC detects that the reaction is complete; wash the reaction solution into a 100mL egg-shaped bottle with ethyl acetate, add an appropriate amount of silica gel, spin dry, and separate by silica gel column chromatography , 78.1mg of yellow solid was obtained, the yield was 94%. Melting point, 181-183°C; 1 HNMR (400MHz, CDCl 3 )δ8.31(s,1H),8.26(s,1H),7.77-7.75(m,2H),7.68-7.59(m,7H),7.53(d,J=6.0Hz,2H),7.47(d ,J=8.0Hz,2H),7.29-7.20(m,4H). 13 CNMR (100.6MHz, CDCl 3 )δ139.11,137.73,137.47,135.40,133.65,132.88,131.44,131.07,130.95,129.3...
Embodiment 3
[0036] Example 3: Synthesis of 5-phenyl-12-(p-trifluoromethylphenyl)naphthacene (5-phenyl-12-(4-(trifluoromethyl)phenyl)tetracene)
[0037]
[0038] Under argon protection, bispropargyl carbonate 1a (0.2mmol, 91mg), p-trifluoromethylphenylboronic acid (0.8mmol, 152mg) and tetrahydrofuran (5mL) were successively added into a 25mL Schlenk reaction tube with a stirrer to dissolve , add tetrakistriphenylphosphine palladium (0.01mmol, 12mg), react at 100°C for 3h, TLC detects that the reaction is complete; wash the reaction solution into a 100mL egg-shaped bottle with ethyl acetate, add an appropriate amount of silica gel, spin dry, and use silica gel column After chromatographic separation, 69.2 mg of yellow solid was obtained, with a yield of 77%. Melting point, 181-182°C; 1 HNMR (400MHz, CDCl 3 )δ8.32(s,1H),8.18(s,1H),7.92(d,J=8.0Hz,2H),7.79-7.76(m,2H),7.70-7.52(m,9H),7.30-7.23 (m,4H). 13 CNMR (100.6MHz, CDCl 3 )δ143.37(q, 5 J C-F =1.1Hz),139.02,137.84,135.01,131.96,13...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com