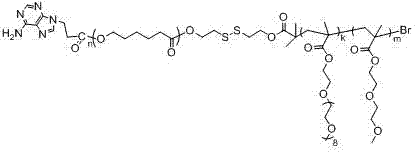
Preparation method of base pair-containing multiple responsive polymer
A polymer and responsive technology, which is applied to non-active ingredients in medical preparations, pharmaceutical formulations, etc., can solve the problem of relatively few studies on the responsiveness of base salts, and achieve the effect of simple and easy synthesis methods and a wide range of sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Dissolve 3.16g of 2-bromoisobutyric acid in 50ml of dichloromethane, and drop into 250ml of dichloromethane dissolved in 5.0g of 2-hydroxyethyl sulfide and 4.7g of hexylcarbodiimide In methane, the system was reacted at room temperature for 12 hours, filtered, rotary evaporated, and dried to obtain product A. Then 0.54g of product A, 6.1636g of caprolactone, and 108 μl of catalyst were dissolved in 2ml of toluene, and the system was reacted at 115°C for 24 hours under the protection of nitrogen. Precipitation gave product B after drying. Dissolve 2 g of product B in 6 ml of dry N,N-dimethylformamide, add 4.649 g of methyl-2-acrylate-2-(2-methoxyethoxy)ethyl ester and 1.021 g of Oligoethylene glycol methyl ether methacrylate, 78.5mg of cuprous bromide, 0.2 mL of hexamethyltriethylenetetramine, the system was reacted at 60°C for 9 hours under the protection of nitrogen, and the reaction was completed. Catalyst, dialyzed, and freeze-dried to obtain product C. 2.327g of ...
Embodiment 2
[0031]Dissolve 2.16g of 2-bromoisobutyric acid in 40ml of dichloromethane, dropwise into 100ml of dichloromethane dissolved in 3.0g of 2-hydroxyethyl sulfide and 3.0g of hexylcarbodiimide In methane, the system was reacted at room temperature for 16 hours, filtered, rotary evaporated, and dried to obtain product A. Then 0.36g of product A, 4.1090g of caprolactone, and 75μl of catalyst were dissolved in 1ml of toluene, and the system was reacted at 130°C for 36 hours under the protection of nitrogen. Precipitation gave product B after drying. Dissolve 1.8 g of product B in 4 ml of dry N,N-dimethylformamide, add 3.630 g of methyl-2-acrylate-2-(2-methoxyethoxy) ethyl ester and 0.798 g oligoethylene glycol methyl ether methacrylate, 78.5mg of cuprous bromide, 0.2 mL of hexamethyltriethylenetetramine, the system was reacted at 80°C for 12 hours under the protection of nitrogen, and the reaction was completed The catalyst was removed, dialyzed, and freeze-dried to obtain product C...
Embodiment 3
[0035] Dissolve 2.56g of 2-bromoisobutyric acid in 30ml of dichloromethane, dropwise into 100ml of dichloromethane dissolved in 3.5g of 2-hydroxyethyl sulfide and 2.7g of hexylcarbodiimide In methane, the system was reacted at 30°C for 12 hours, filtered, rotary evaporated, and dried to obtain product A. Then 0.50 g of product A, 5.27 g of caprolactone, and 90 μl of catalyst were dissolved in 2 ml of toluene, and the system was reacted at 115° C. for 24 hours under the protection of nitrogen. After the reaction was completed, it was diluted with tetrahydrofuran and precipitated with ether. Product B was obtained after drying. Dissolve 2 g of product B in 6 ml of dry N,N-dimethylformamide, add 2.420 g of methyl-2-acrylate-2-(2-methoxyethoxy)ethyl ester and 0.552 g of Oligoethylene glycol methyl ether methacrylate, 78.5mg of cuprous bromide, 0.3 mL of hexamethyltriethylenetetramine, the system was reacted at 60°C for 9 hours under the protection of nitrogen, and the reaction wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com