Alpha amylase and aspergillus niger strain for expressing same
A technology of α-amylase and Aspergillus, which is applied in the field of filamentous fungal host Aspergillus niger cells, can solve the problems of low expression of production strains and restrict the wide application of amylase, and achieve the effect of broadening the market space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
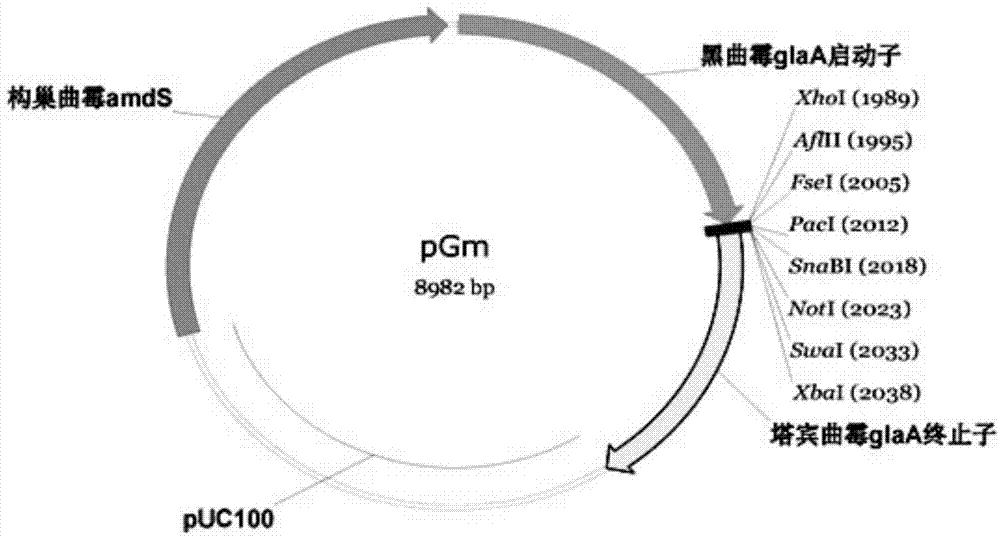
[0073] Cloning of Example 1 α-amylase gene AclaP11
[0074] Genomic DNA was extracted from overnight cultures of Aspergillus clavus CGMCC3.5289 using the Fungal Genomic DNA Extraction Kit (Omega) following the manufacturer's instructions.
[0075] Design PCR primers according to the α-amylase gene sequence of XM_001271888 on the NCBI, and the sequence of the forward primer AclaP11-F for cloning the AclaP11 gene in Aspergillus clavus is as follows:
[0076] 5'-ACGGC CTTAAG AAGATGCCTCGGATTTGGTCCTC
[0077] (the sequence shown in the underline is AflII restriction site),
[0078] The reverse primer AclaP11-R sequence is:
[0079] 5'-ATT GCGGCCGC AGCTCAAAGCACAGATCTTGCTTC
[0080] (The underlined sequence is the NotI restriction site).
[0081] The gene was amplified from A. claviculare genomic DNA using Phusion DNA polymerase (Thermo scientific).
[0082] Amplification conditions:
[0083] Step 1: Keep at 98°C for 3 minutes.
[0084] The second step: hold at 98°C for 10...
Embodiment 2
[0088] Embodiment 2: PEG-mediated protoplast fusion transforms Aspergillus niger
[0089]Draw the Aspergillus niger G1 spore suspension in the center of the CMA plate (9cm petri dish), wait for the colony to cover the whole petri dish, cut 1 / 4 size of the culture based on 200mL CMA liquid medium, culture at 30°C, 200rpm for 14~ 16h.
[0090] Collect the mycelium with a sterile Miracloth filter cloth, and wash it once with solution A, transfer the washed mycelium to 40mL protoplastization solution under aseptic conditions, and incubate at 30°C and 200rpm for 1-2h, The progress of protoplastization was detected by microscopic observation.
[0091] Filter the above-mentioned warm bath liquid with a sterile Miracloth filter cloth, and the obtained filtrate is the protoplast solution. The protoplast solution was divided into two 50mL sterile disposable centrifuge tubes, and the volume of each tube was adjusted to 45mL with solution B, centrifuged at 4000rpm for 8min to obtain the...
Embodiment 3
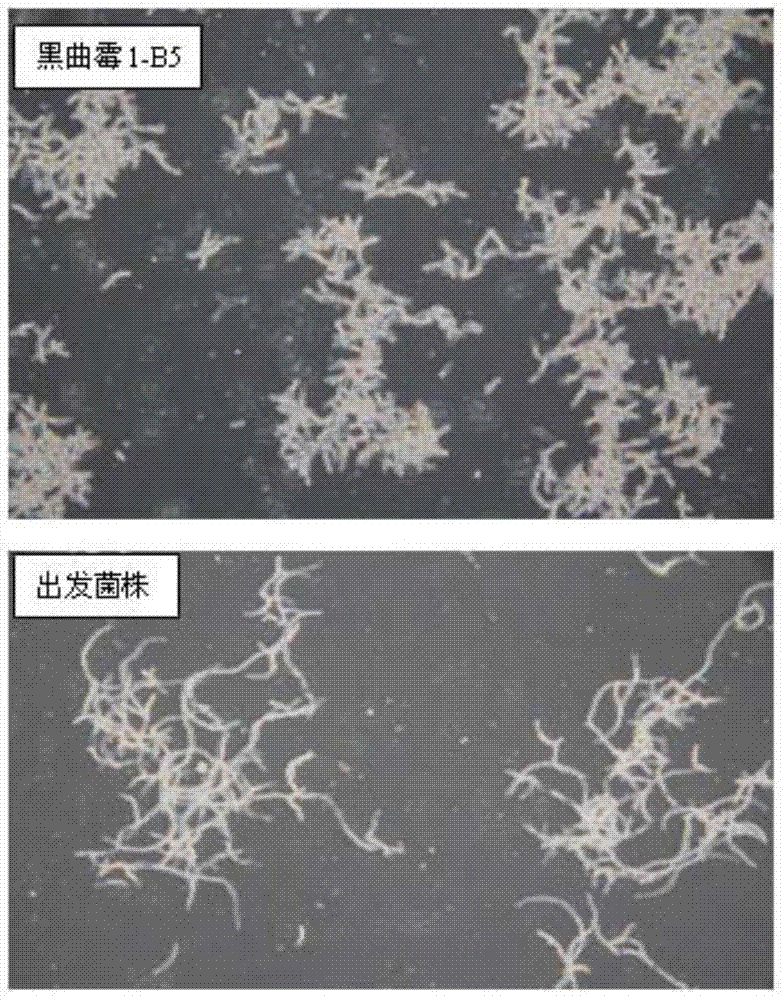
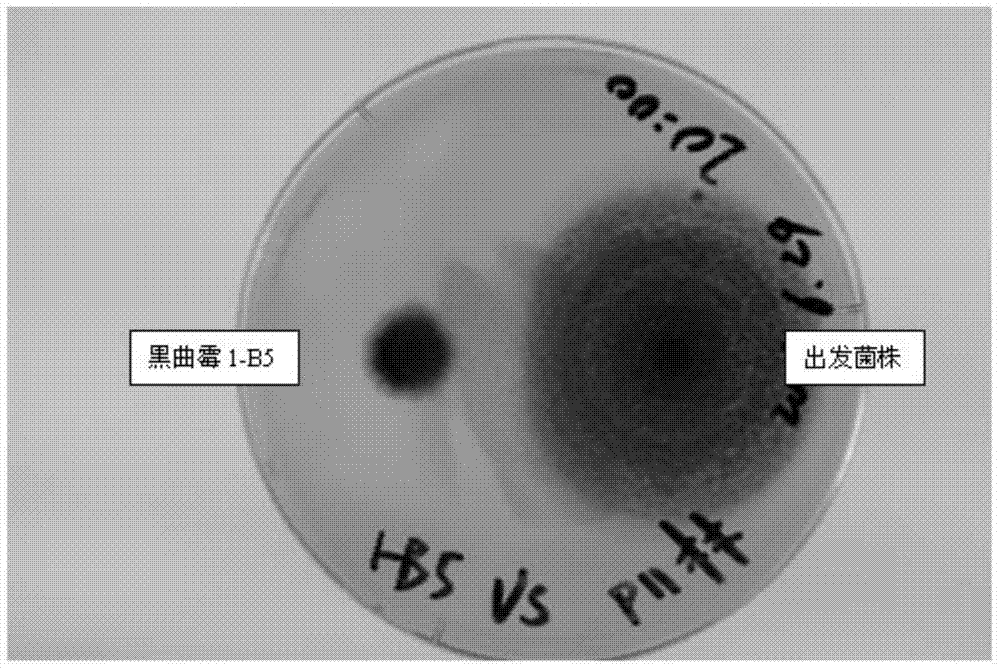
[0104] Embodiment 3: the ultraviolet mutagenesis and screening of starting bacterial strain Aspergillus niger 1-B
[0105] Aspergillus niger 1-B was used as the starting strain for ultraviolet mutagenesis and screening.
[0106] Inoculate the starting strain Aspergillus niger 1-B on CMA solid medium, culture at 30°C for 5 days until black spores grow, wash the spores with sterile water, and dilute to a spore concentration of 10 6 level, the spore suspension was irradiated under an ultraviolet lamp for 4 minutes, the irradiation distance was 22 cm, and the power of the ultraviolet lamp was 30w. The resulting spore suspension was diluted 100 times, coated on a CMA solid medium plate, and after 48 to 72 hours, a small and dense colony with a small colony radius was selected, streaked and inoculated on a CMA solid medium plate, and after it grew black spores, use After washing with sterile water, inoculate in TSB fermentation medium, measure enzyme activity after 5 days of fermen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com