Method for preparing phosphite ester compounds through reactive rectification coupling
A technology of phosphite and reactive distillation, which is applied in the field of chemical production technology, can solve the problems of increased production cost, sedimentation blockage, viscosity increase, etc., and achieve the effects of increasing production rate, avoiding blockage of equipment, and speeding up the reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
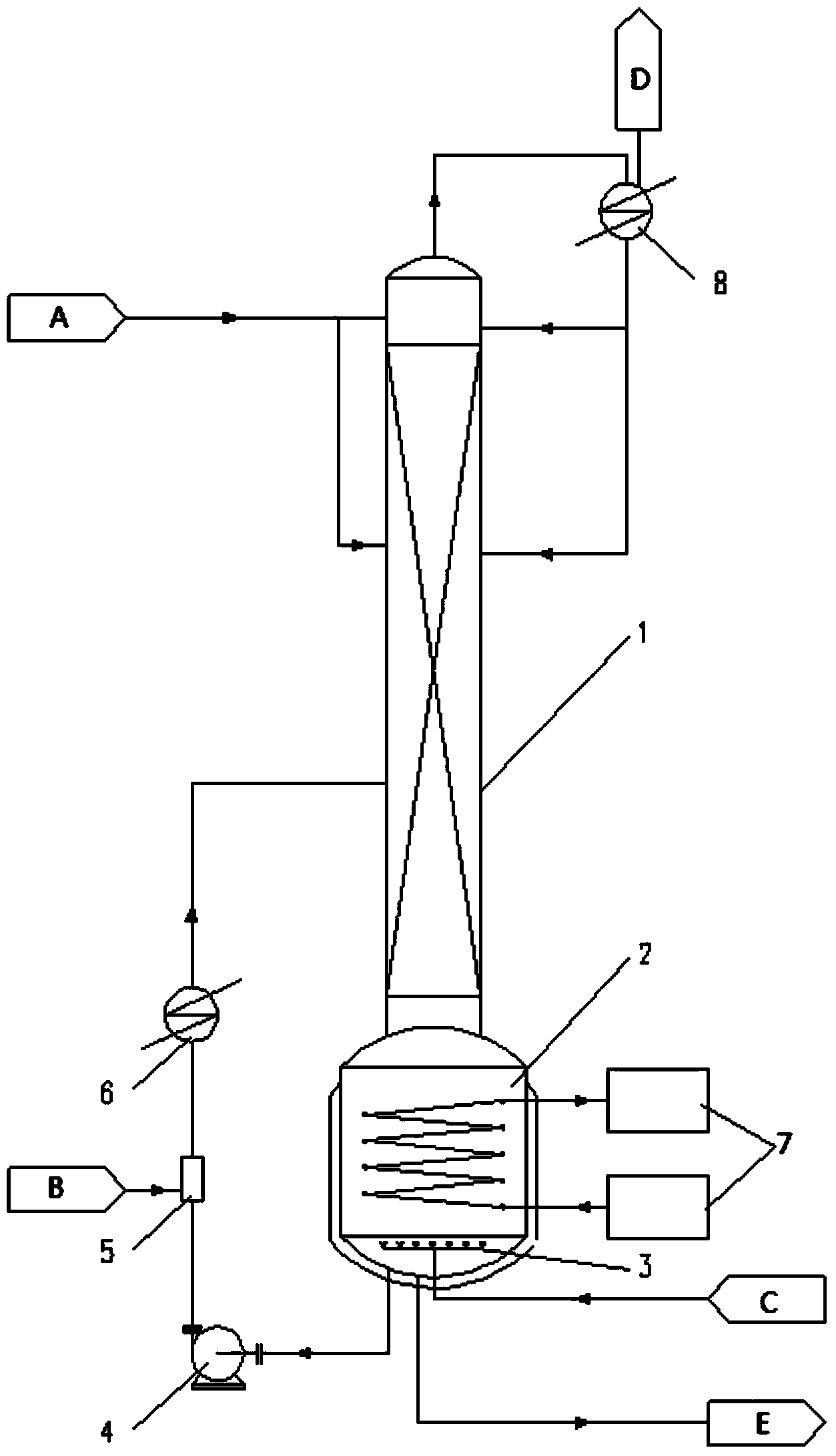
[0046] 10kg of phenol (reactant 1A) was added to the reactor (2). 4.42 kg of phosphorus trichloride (reactant 2B) was sent to the mixer (5) by metering pump. The temperature of the reaction kettle (2) is 160°C, and the pressure is 1KPag. The feed rate of phosphorus trichloride (reactant 2B) is 1 l / h, and the circulation flow rate of the circulation pump (4) is 12 l / h. The inert gas (C) is high-purity nitrogen with a flow rate of 0.5kg / h, which is fed into the reactor (2) through the sparger (3). The condensing temperature of the heat exchanger 2 (8) is 35° C., and the condensate is refluxed to the top of the tower (1). After reacting for 6 hours, samples were taken for gas chromatography analysis, and the conversion rate of phosphorus trichloride (reactant 2B) was 100%, and the yield of triphenyl phosphite (E) was 94%.
Embodiment 2
[0048] 10kg of phenol (reactant 1A) was added to the reactor (2). 4.42 kg of phosphorus trichloride (reactant 2B) was sent to the mixer (5) by metering pump. The temperature of the reaction kettle (2) is 180°C, and the pressure is 1KPag. The feed rate of phosphorus trichloride (reactant 2B) is 1 l / h, and the circulation flow rate of the circulation pump (4) is 20 l / h. The inert gas (C) is high-purity nitrogen with a flow rate of 1kg / h, which is fed into the reactor (2) through the distributor (3). The condensing temperature of the heat exchanger 2 (8) is 35° C., and the condensate is refluxed to the top of the tower (1). After reacting for 6 hours, samples were taken for gas chromatography analysis, and the conversion rate of phosphorus trichloride (reactant 2B) was 100%, and the yield of triphenyl phosphite (E) was 90%.
Embodiment 3
[0050] 10kg m-cresol (reactant 1A) was added to the reactor (2). 3.84 kg of phosphorus trichloride (reactant 2B) was sent to the mixer (5) by metering pump. The temperature of the reaction kettle (2) is 150°C, and the pressure is 1KPag. The feed rate of phosphorus trichloride (reactant 2B) is 1 l / h, and the circulation flow rate of the circulation pump (4) is 20 l / h. The inert gas (C) is high-purity nitrogen with a flow rate of 0.5kg / h, which is fed into the reactor (2) through the distributor (3). The condensing temperature of the heat exchanger 2 (8) is 35° C., and the condensate is refluxed to the top of the tower (1). After reacting for 10 hours, samples were taken for gas chromatography analysis, the conversion rate of phosphorus trichloride (reactant 2B) was 100%, and the yield of tri-m-cresyl phosphite (E) was 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com