Method for separating and preparing anti-tumor components ganoderic acid C1 and ganoderic acid F
A technology of ganoderma acid and anti-tumor is applied in the field of separation and preparation of high-purity anti-tumor components ganoderma acid C1 and ganoderma acid F, which can solve the problems of pollution, increase of test cost, waste of resources and environment, etc., so as to improve the efficiency of separation and purification and save the test. cost, the effect of eliminating environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
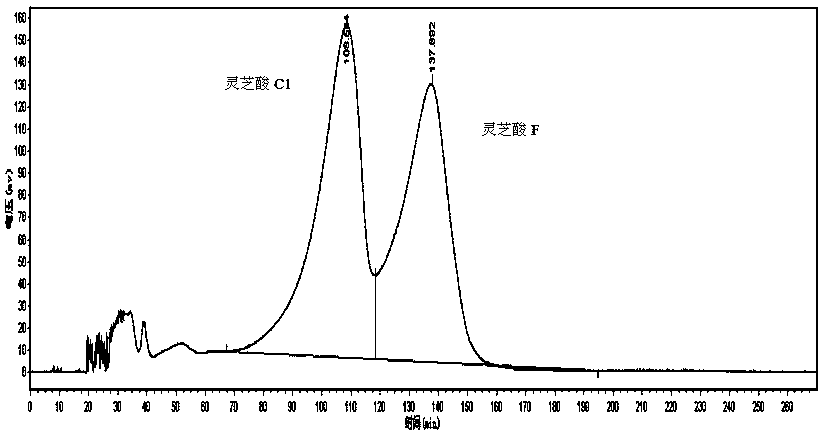
[0027] 1.1 Macroporous resin separation: Dissolve the ethanol extract of Ganoderma lucidum with 40% ethanol, filter and separate on macroporous resin HZ-816 column, and carry out gradient elution with water, 30%, 50%, 70% and 90% ethanol in sequence. 1 / 6 column volume is collected as a fraction, and the fraction is analyzed by liquid phase to detect its composition, and the fractions mainly containing ganoderma acid C1 and ganoderma acid F are combined, concentrated to obtain a mixture mainly composed of ganoderma acid C1 and ganoderma acid F, high-efficiency liquid Phase chromatograph analysis detects that its purity is 39.5%.
[0028]1.2 High-speed countercurrent separation: TBE-300B semi-preparative high-speed countercurrent chromatography is used. According to the solvent system, petroleum ether: ethyl acetate: methanol: water = 5:5:3.5:6.5, the solution was formulated into a separatory funnel, shaken well, left to stand for layering, and the upper and lower layer solution...
Embodiment 2
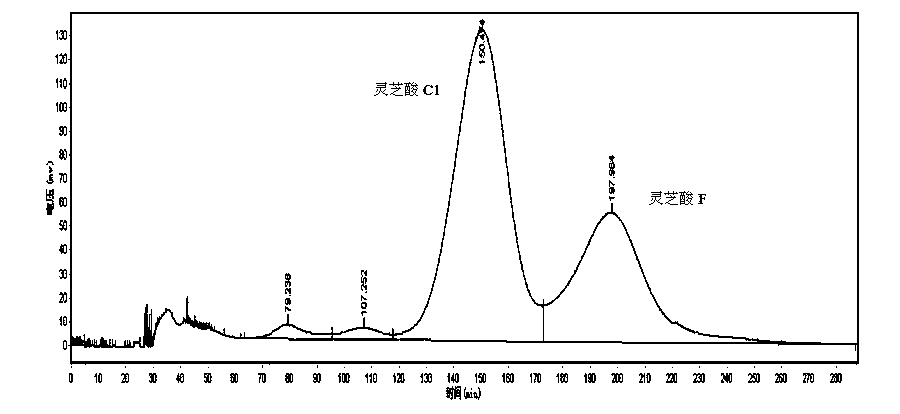
[0030] 2.1 Macroporous resin separation: Dissolve the ethanol extract of Ganoderma lucidum in 45% ethanol, filter and separate on macroporous resin HZ-818 column, wash with water, 30%, 45%, 60%, 75%, 90% ethanol in sequence Each 1 / 5 column volume is collected as a fraction, and the fraction is analyzed by HPLC to detect its composition, and the fractions containing ganoderma acid C1 and ganoderma acid F are combined and concentrated to obtain ganoderma acid C1 and ganoderma acid F, with a purity of 42.8%.
[0031] 2.2 High-speed countercurrent separation: TBE-300B semi-preparative high-speed countercurrent chromatography is used. Prepare the solution according to the solvent system of petroleum ether-ethyl acetate-methanol-water=5:5:3:7 in the separatory funnel, shake it well, let it stand for stratification, separate the upper and lower layer solutions and ultrasonically degas; the above The phase is the stationary phase, and the lower phase is the mobile phase. The stationar...
Embodiment 3
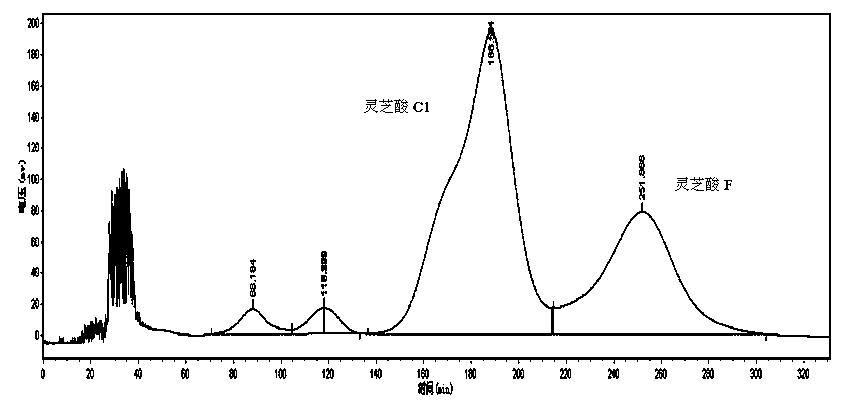
[0033] 3.1 Macroporous resin separation: Dissolve the ethanol extract of Ganoderma lucidum in 50% ethanol, filter and separate on macroporous resin HZ-816 column, wash with water, 30%, 45%, 60%, 75%, 90% ethanol in sequence Each 1 / 8 column volume is collected as a fraction, and the composition of the fraction is detected by liquid phase analysis. The fractions containing ganoderma acid C1 and ganoderma acid F are combined and concentrated to obtain a mixture of ganoderma acid C1 and ganoderma acid F with a purity of 47.7%.
[0034] 3.2 High-speed countercurrent separation: TBE-300B semi-preparative high-speed countercurrent chromatography is used. According to the solvent system of petroleum ether-ethyl acetate-methanol-water=5:5:2.5:7.5, prepare the solution in the separatory funnel, shake it well, let it stand for stratification, separate the upper and lower layer solutions and ultrasonically degas; the above The phase is the stationary phase, and the lower phase is the mobi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com