Novel ruthenium complex and method for preparing methanol and diol
A technology for ruthenium complexes and diols, applied in the field of preparing methanol and diols, can solve the problems that metal complexes have no catalytic activity, carbon dioxide has not been effectively utilized, and have no practicality and economic value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
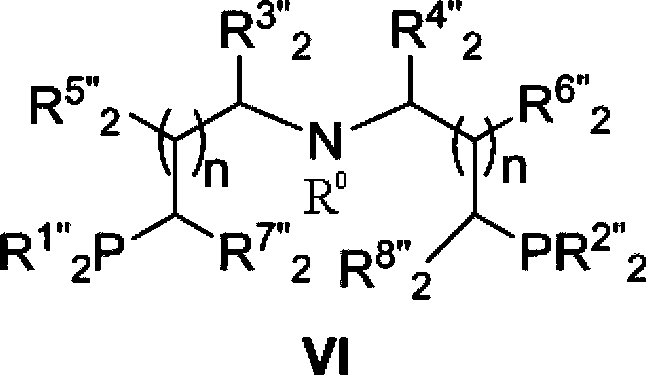
[0139] The preparation method of catalyst of the present invention
[0140] Ruthenium catalyst of the present invention can be prepared by following method:
[0141] Under an inert gas atmosphere, such as nitrogen or argon, at 60-120°C, 1 equivalent of a ruthenium metal precursor, such as [RuHCl(CO)(PPh 3 ) 3 ], [RuH 2 (CO)(PPh 3 ) 3 ], RuCl 2 (PPh 3 ) 3 , [RuCl 2 (C 6 h 6 )] 2 , [RuHCl(PPh 3 ) 3 ], Ru(DMSO) 4 Cl 2 , [Ru(cod)Cl 2 ], [Ru(nbd)Cl 2 ] and 1 to 1.2 equivalents of tridentate aminobisphosphorus ligand reacted in a solvent for 0.5-20h in the system. Wherein said DMSO represents dimethyl sulfoxide, cod represents 1,5-cyclooctadiene, and nbd represents norbornadiene.
[0142] The application of catalyst of the present invention
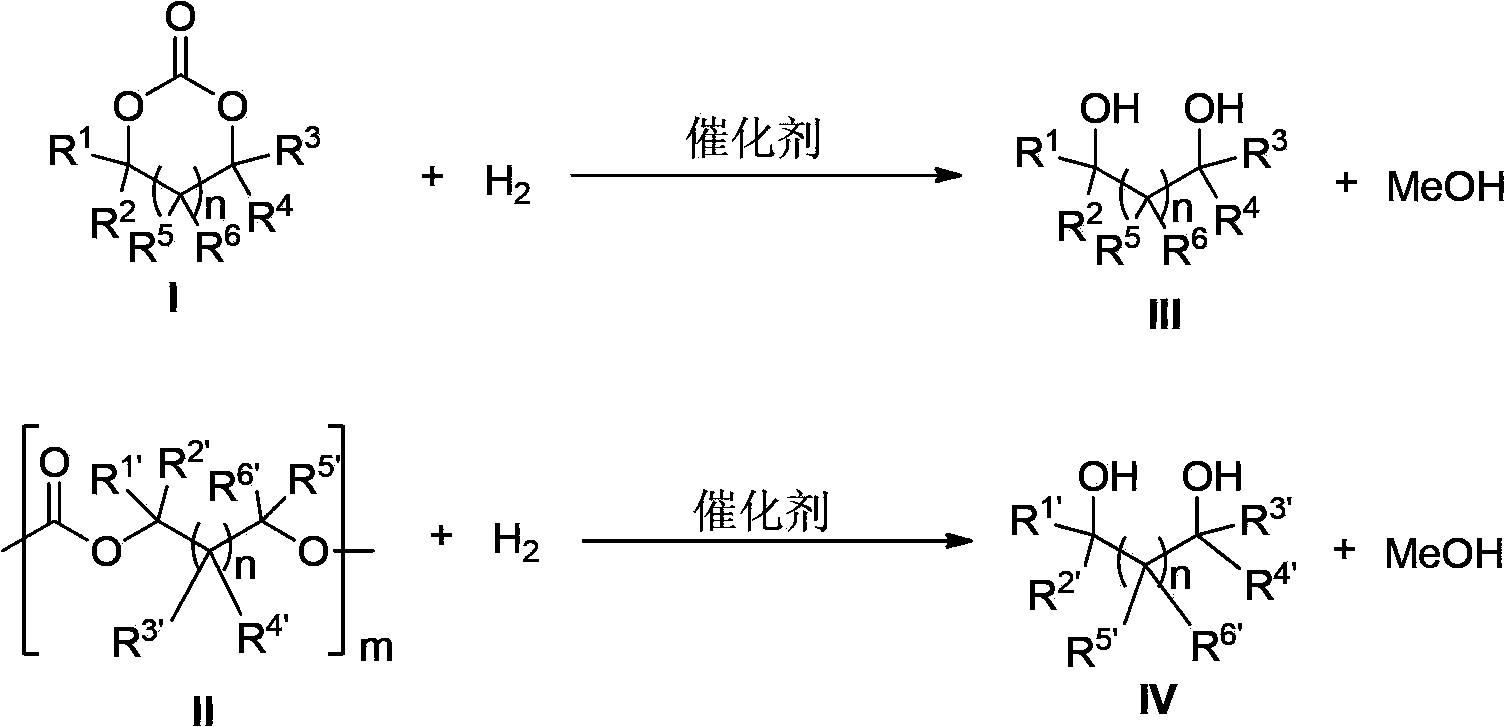
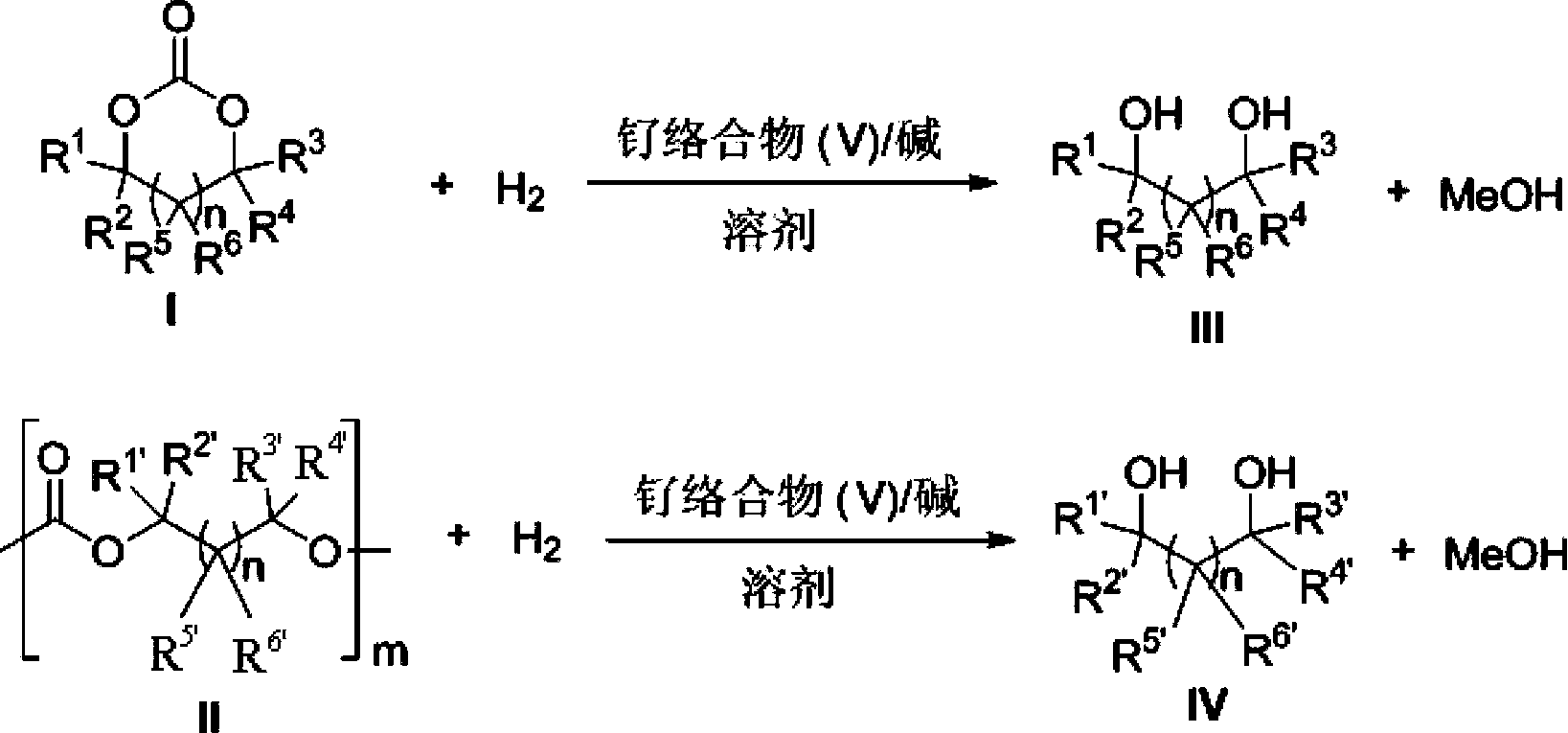
[0143] Catalyst of the present invention, i.e. ruthenium complex can be used for carbonate, comprise cyclic carbonate, polycarbonate and straight-chain carbonate hydrogenation reduction to methanol and other corresponding alc...
Embodiment 1
[0169] Embodiment 1: the preparation of catalyst ruthenium complex 1a
[0170]
[0171] Under an inert gas atmosphere such as nitrogen or argon, add HCl·HN(CH 2 CH 2 PPh 2 ) 2 (1.20g, 2.51mmol), toluene (20mL) and 15% aqueous sodium hydroxide solution (10mL). The reaction mixture was stirred at room temperature until the solids were completely dissolved. The organic phase was separated under inert atmosphere and washed twice with distilled water (2 x 5 mL). The combined aqueous phases were extracted twice with toluene (2 x 10 mL). All organic phases were combined and dried over anhydrous sodium sulfate. After removing the desiccant by filtration, the solvent was removed under reduced pressure in vacuo to obtain the free aminobisphosphorus ligand crude product. This crude product was dissolved in toluene (18 mL) and RuHCl(CO) (PPh 3 ) 3 (2.28g, 2.39mmol), reflux reaction for 2 hours. After the reaction system was cooled to room temperature, hexane (10 mL) was added...
Embodiment 2
[0173] Embodiment 2: the preparation of catalyst ruthenium complex 1b
[0174]
[0175] HN(CH 2 CH 2 P i PR 2 ) 2 (217mg, 0.710mmol) and RuHCl(CO)(PPh 3 ) 3 (644mg, 0.676mmol) was dissolved in toluene (4mL), heated to reflux for 5 hours. After the reaction solution was cooled to room temperature, hexane (6 mL) was added. The precipitated solid was filtered and dried to obtain 288 mg of ruthenium complex 1b with a yield of 90%.
[0176] 1 H NMR (400MHz, CDCl 3 )δ3.50-3.39(m,1H),3.31-3.26(m,2H),2.77-2.65(m,2H),2.35-2.09(m,6H),1.86-1.74(m,2H),1.60- 1.44(m,6H),1.34-1.08(m,18H),-16.30(t,J=19.2Hz,0.12H),-16.54(t,J=18.0Hz,0.88H)ppm; 31 P NMR (161.9MHz, CDCl 3 )δ74.6(s,br)ppm; HRMS(MALDI)m / z calcd.for C 17 h 38 NOP 2 96 Ru:430.1499, Found:430.1502[M-Cl] + ;IR (film) 1973, 1960, 1910cm -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com